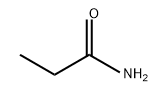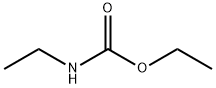
ETHYL N-ETHYLCARBAMATE synthesis
- Product Name:ETHYL N-ETHYLCARBAMATE
- CAS Number:623-78-9
- Molecular formula:C5H11NO2
- Molecular Weight:117.15

64-17-5
825 suppliers
$10.00/50g
51-79-6
453 suppliers
$13.00/25g
75-13-8
103 suppliers
inquiry

60-29-7
0 suppliers
$30.00/50g

623-78-9
30 suppliers
inquiry
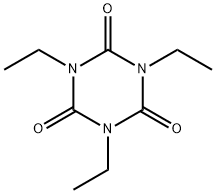
715-63-9
1 suppliers
inquiry
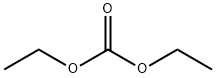
105-58-8
480 suppliers
$14.00/25g
Yield:-
Reaction Conditions:
dibutyldimethoxytin in Triethylene glycol dimethyl ether at 173.879; under 4173.32 Torr; for 340 h;
Steps:
5
The purpose of this experiment is demonstrating the production of diethyl carbonate (DEC). DEC was produced by reacting ethyl carbamate (EC) with ethanol. The experiment was carried out in the similar manner to the Example 2. An ethyl carbamate solution in ethanol and ethanol were used in place of MC solution and methanol respectively in Example 2. The reboiler of the distillation column was loaded with the following materials; 180 grams of triglyme, 100 grams of ethanol and 100 grams of dibutyltin dimethoxide. A steady state operation of the distillation column reactor was obtained, while pumping in an ethyl carbamate (EC) solution at a constant flow rate and adjusting the ethanol pumping rate to maintain a constant temperature of the liquid reaction medium. The reactor operation was continued for 340 hours without interruption at 345° F. of the liquid reaction medium in the reboiler, the distillation column temperature of 282° F., and a constant overhead pressure of 66 psig with an autoclave stirring rate of 300 rpm. The pumping rate of a 15.35 wt. % EC solution (275 ppm H2O) was fixed at 2.69 ml/min and the average pumping rate of ethanol (106 ppm H2O) was 2.36 ml/min. The overhead vapor stream at the top of the column was mixed with nitrogen dilution gas (600 cc/min) and then cooled to about 200° F. in a water cooled condenser. The average compositions of the overhead products and the composition of the bottom products for the entire run are listed in Table 3. 74.2 grams of solid material were removed from the reactor at the end run, which was a mixture of heterocyclic compounds and contained 670 ppm Sn by weight. The analysis of the bottom product in Table 3 indicates that the mole ratio of EC/C2H5OH and DEC wt. % based on EC and ethanol in the liquid medium in the reactor was 0.939 and 11.08 wt. % respectively. The mass balance and urea mole balance for the entire run were 102% and 101%, respectively. The run result indicates 57.5% conversion of EC and 91 mole % selectivity of EC to DEC. The experimental result translates to DEC space yield of 1.60 lb/h/ft3.
References:
CATALYTIC DISTILLATION TECHNOLOGIES US2005/203307, 2005, A1 Location in patent:Page/Page column 10; 11

64-17-5
825 suppliers
$10.00/50g

51-79-6
453 suppliers
$13.00/25g

623-78-9
30 suppliers
inquiry

105-58-8
480 suppliers
$14.00/25g

124-09-4
390 suppliers
$14.00/5g

64-17-5
825 suppliers
$10.00/50g

51-79-6
453 suppliers
$13.00/25g
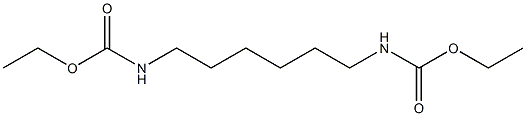
3066-65-7
13 suppliers
$29.00/1g

623-78-9
30 suppliers
inquiry

105-58-8
480 suppliers
$14.00/25g
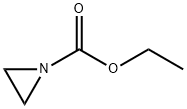
671-51-2
12 suppliers
inquiry

623-78-9
30 suppliers
inquiry