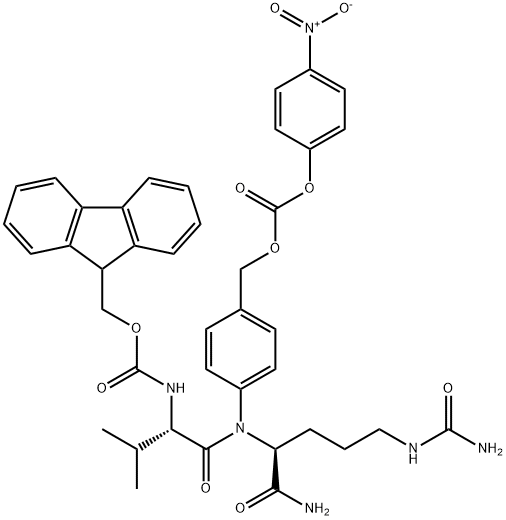
FMoc-Val-Cit-PAB-PNP synthesis
- Product Name:FMoc-Val-Cit-PAB-PNP
- CAS Number:863971-53-3
- Molecular formula:C40H42N6O10
- Molecular Weight:766.8
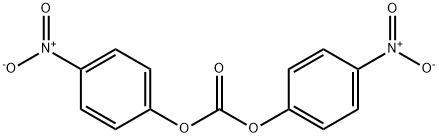
5070-13-3
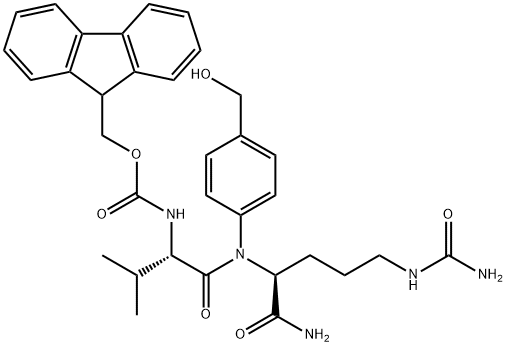
159858-22-7

863971-53-3
General procedure for the synthesis of compound (CAS:863971-53-3) from bis(4-nitrophenyl) carbonate and compound (CAS:159858-22-7): under nitrogen protection, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-valyl-N5-carbamoyl-N-[4-(hydroxymethyl)phenyl]-L-ornithinamide ( 1.3 g, 2.16 mmol, prepared as reported in EP0624377A2) was dissolved in 6 mL of anhydrous DMF with bis(4-nitrophenyl) carbonate (1.32 g, 4.34 mmol). After addition of DIPEA (0.75 mL, 4.35 mmol), the reaction mixture was stirred at room temperature for 1 hour. After completion of the reaction, diethyl ether (120 mL) was added to precipitate the product, and the precipitate was collected by filtration, washed with diethyl ether, and dried under vacuum to afford N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-valyl-N5-carbamoyl-N-[4-({{[(4-nitrophenoxy)carbonyl]oxy}methyl)phenyl]-L-ornithinamide (1.47 g, yield 89%). ESI MS: m/z 767 (MH+). 1H NMR (400MHz, DMSO-d6) δ 0.86 (d, J=6.7Hz, 3H), 0.88 (d, J=6.7Hz, 3H), 1.30-1.52 (m, 2H), 1.60 (m, 1H), 1.69 (m, 1H), 1.99 (m, 1H ), 2.90-3.10 (m, 2H), 3.93 (dd, J=8.9,7.0Hz, 1H), 4.14-4.34 (m, 3H), 4.42 (m, 1H), 5.24 (s, 2H), 5.39 (s, 2H), 5.97 (t, J=5.5Hz, 1H), 7.32 (m, 2H), 7.42 (m, 5H ), 7.55 (m, 2H), 7.65 (d, J=8.4Hz, 2H), 7.74 (t, J=7.9Hz, 2H), 7.88 (d, J=7.6Hz, 2H), 8.12 (d, J=7.4Hz, 1H), 8.31 (m, 2H), 10.12 (s, 1H).

5070-13-3
254 suppliers
$5.00/5g

159858-22-7
222 suppliers
$14.00/25MG

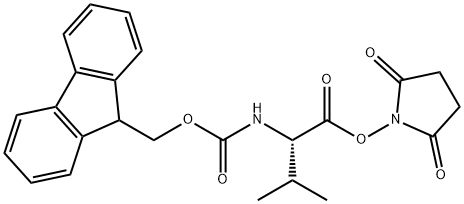
863971-53-3
209 suppliers
$16.00/1mg
Yield:863971-53-3 89%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20; for 1 h;Inert atmosphere;
Steps:
a
N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-valyl-N5-carbamoyl-N-[4-(hydroxymethyl)phenyl]-L-ornithinamide (1.3 g, 2.16 mmol) (prepared as reported in EP0624377A2) and bis(4-nitrophenyl) carbonate (1.32 g, 4.34 mmol) were dissolved in 6 mL of dry DMF under nitrogen atmosphere, DIPEA (0.75 mL, 4.35 mmol) was added and the resulting solution was stirred an hour at room temperature. Diethylether (120 mL) was added, the resulting precipitate is filtered off, washed with diethylether and dried under vacuum affording N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-valyl-N5- carbamoyl-N-[4-({[(4-nitrophenoxy)carbonyl]oxy}methyl)phenyl]-L-ornithinamide (1.47 g, 89% yield).ESI MS: m/z 767 (MH+)1H NMR (400 MHz, DMSO-de) δ 0.86 (d, J = 6.7 Hz, 3 H), 0.88 (d, J = 6.7 Hz, 3 H), 1.30 - 1.52 (m, 2 H), 1.60 (m, 1 H), 1.69 (m, 1 H), 1.99 (m, 1 H), 2.90 - 3.10 (m, 2 H), 3.93 (dd, J = 8.9, 7.0 Hz, 1 H), 4.14 - 4.34 (m, 3 H), 4.42 (m, 1 H), 5.24 (s, 2 H), 5.39 (s, 2 H), 5.97 (t, J = 5.5 Hz, 1 H), 7.32 (m, 2 H), 7.42 (m, 5 H), 7.55 (m, 2 H), 7.65 (d, J = 8.4 Hz, 2 H), 7.74 (t, J = 7.9 Hz, 2 H), 7.88 (d, J = 7.6 Hz, 2 H), 8.12 (d, J = 7.4 Hz, 1 H), 8.31 (m, 2 H), 10.12 (s, 1 H)
References:
NERVIANO MEDICAL SCIENCES S.R.L.;CALDARELLI, Marina;CARUSO, Michele;ORSINI, Paolo WO2015/44003, 2015, A1 Location in patent:Page/Page column 39
7693-46-1
433 suppliers
$18.00/5g

159858-22-7
222 suppliers
$14.00/25MG

863971-53-3
209 suppliers
$16.00/1mg

67-56-1
790 suppliers
$7.29/5ml-f

5070-13-3
254 suppliers
$5.00/5g

159858-22-7
222 suppliers
$14.00/25MG

863971-53-3
209 suppliers
$16.00/1mg
130878-68-1
156 suppliers
$5.00/1g

863971-53-3
209 suppliers
$16.00/1mg
68858-20-8
543 suppliers
$8.00/1g

863971-53-3
209 suppliers
$16.00/1mg