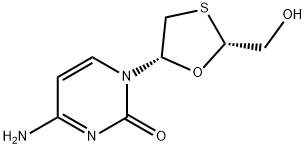
Lamivudine synthesis
- Product Name:Lamivudine
- CAS Number:134678-17-4
- Molecular formula:C8H11N3O3S
- Molecular Weight:229.26
173522-96-8
82 suppliers
$49.00/25mg

134678-17-4
704 suppliers
$5.00/25mg
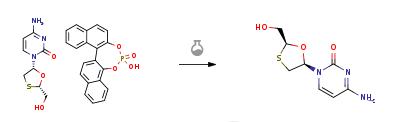
Yield:134678-17-4 92.63%
Reaction Conditions:
with triethylamine in ethanol at 50; for 1 h;
Steps:
1.7 7) Preparation of lamivudine:
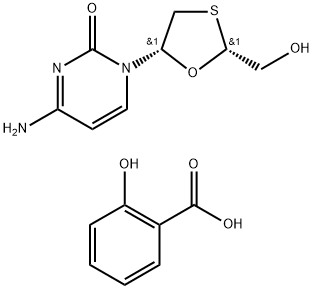
Add 38.5g (0.1mol) lamivudine salicylate,320ml of absolute ethanol and 15.15g (0.15mol) of triethylamine,The temperature was raised to 50 ° C, and the reaction was held for 1 h.After the lamivudine salicylate is sufficiently recrystallized,The solvent was distilled off under reduced pressure, 300 ml of ethyl acetate was added, and the mixture was cooled to 10 ° C and kept under stirring for 1 hour.Filtered, washed twice with 100 ml ethyl acetate,Dried to give 21.21g of lamivudine as a white powder,After calculation, the yield is 92.63%.
References:
CN110437216,2019,A Location in patent:Paragraph 0028; 0036; 0038
![Carbonic acid, [(2R,5S)-5-(4-amino-2-oxo-1(2H)-pyrimidinyl)-1,3-oxathiolan-2-yl]methyl [(1R,2S,5R)-5-methyl-2-(1-methylethyl)cyclohexyl] ester](/CAS/20210305/GIF/1012053-56-3.gif)
1012053-56-3
0 suppliers
inquiry

134678-17-4
704 suppliers
$5.00/25mg
![(1R,2S,5R)-Menthyl-(2R,5S)-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-[1,3]oxathiolane-2-carboxylic acid](/CAS/GIF/147027-10-9.gif)
147027-10-9
165 suppliers
$98.00/1g
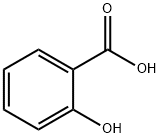
69-72-7
1312 suppliers
$5.00/10g

134678-17-4
704 suppliers
$5.00/25mg
![(1R,2S,5R)-Menthyl-(2R,5S)-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-[1,3]oxathiolane-2-carboxylic acid](/CAS/GIF/147027-10-9.gif)
147027-10-9
165 suppliers
$98.00/1g
2216-51-5
707 suppliers
$5.00/100mg

134678-17-4
704 suppliers
$5.00/25mg
![(1R,2S,5R)-Menthyl-(2R,5S)-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-[1,3]oxathiolane-2-carboxylic acid](/CAS/GIF/147027-10-9.gif)
147027-10-9
165 suppliers
$98.00/1g

134678-17-4
704 suppliers
$5.00/25mg