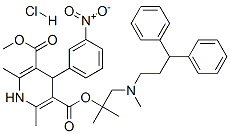
Lercanidipine hydrochloride synthesis
- Product Name:Lercanidipine hydrochloride
- CAS Number:132866-11-6
- Molecular formula:C36H42ClN3O6
- Molecular Weight:648.19
![3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-[2-[(3,3-diphenylpropyl)methylamino]-1,1-dimethylethyl] 5-methyl ester, ethanedioate (1:1)](/CAS/20211123/GIF/957215-03-1.gif)
957215-03-1

132866-11-6
The general procedure for the synthesis of 3-(1-((3,3-diphenylpropyl)(methyl)amino)-2-methylpropan-2-yl)5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate hydrochloride from the compound (CAS:957215-03-1) is as follows: 10 mmol of oxalate salt (Example 4) was dissolved in 100 mL of dichloromethane and 20 mL of aqueous solution containing 20 mmol of potassium carbonate was added. The organic phase was separated and the aqueous phase was extracted with 2 x 10 mL of dichloromethane. The organic phase was combined and treated with 20 mL of 1N hydrochloric acid solution. The organic phase was separated and concentrated. The residue was dissolved in 2,000 mL of water and 2 mL of 1N hydrochloric acid and 5 mL of saturated aqueous sodium chloride were added to form I hydrochloride hemihydrate and the product was collected by filtration. Yield: 6.2 g (95%); Purity: 99.8% (HPLC); Melting point: 118-122 ° C. HPLC conditions: RP 16 column, 125×3 mm ID, 5 μm, Detection wavelength: 233 nm; Eluent composition: 0.01 mol Na2HPO4/MeOH/acetonitrile=25/65/10; Retention time: about 15 min.
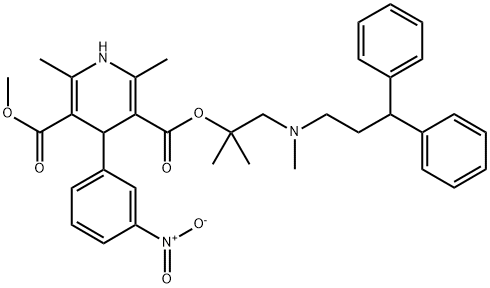
100427-26-7
159 suppliers
inquiry

132866-11-6
380 suppliers
$5.00/1mg
Yield:132866-11-6 90%
Reaction Conditions:
with hydrogenchloride in diethyl ether;tert-butyl methyl etherProduct distribution / selectivity;
Steps:
3B
1.1 eq. of 2N HCl in Et2O was added dropwise under stirring to a solution of 1 g of lercanidipine free base in 20 mL of MTBE. The precipitate formed was filtered under vacuum, rinsed with MTBE and dried under vacuum (0.15 mmHg) at 300C for 4h affording a dry powder (Yield: 90%), HPLC assay: 99,6%.
References:
RECORDATI IRELAND LIMITED WO2008/15248, 2008, A1 Location in patent:Page/Page column 7
21731-17-9
70 suppliers
inquiry

132866-11-6
380 suppliers
$5.00/1mg
![2-[(3-Nitrophenyl)methylene]-3-oxo-butanoic Acid 2-[(3,3-Diphenylpropyl)methylamino]-1,1-dimethylethyl Ester Hydrochloride](/CAS/20200401/GIF/CB75077145.gif)
929212-20-4
3 suppliers
inquiry
14205-39-1
510 suppliers
$6.00/25g

132866-11-6
380 suppliers
$5.00/1mg
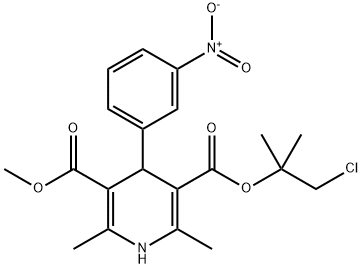
890045-70-2
9 suppliers
inquiry
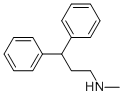
28075-29-8
133 suppliers
inquiry

132866-11-6
380 suppliers
$5.00/1mg
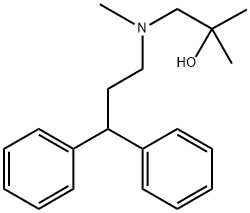
100442-33-9
219 suppliers
$8.00/1g

132866-11-6
380 suppliers
$5.00/1mg