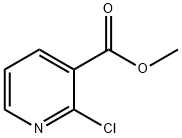
METHYL 2-CHLORONICOTINATE synthesis
- Product Name:METHYL 2-CHLORONICOTINATE
- CAS Number:40134-18-7
- Molecular formula:C7H6ClNO2
- Molecular Weight:171.58

67-56-1
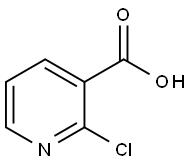
2942-59-8

40134-18-7
(1) Preparation of methyl 2-chloronicotinate: 15.0 g (95.2 mmol) of 2-chloronicotinate was dissolved in 150 mL of dichloromethane and 8.37 mL (94.5 mmol) of oxalyl chloride was added slowly and dropwise. Subsequently, N,N-dimethylformamide was added dropwise to the mixture and the reaction mixture was stirred at room temperature for 3 hours. Upon completion of the reaction, 40.1 mL (286 mmol) of triethylamine and 37 mL (914 mmol) of methanol were added dropwise to the reaction mixture under ice-bath cooling conditions and stirring was continued for 30 minutes under ice-bath cooling. The reaction mixture was concentrated under reduced pressure and neutralized by adding aqueous sodium bicarbonate to the residue. The aqueous phase was extracted with ether, the organic phases were combined, washed with water, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by column chromatography (eluent: ethyl acetate/hexane=1:4) to afford 13.9 g of the target product methyl 2-chloronicotinate as a pale yellow liquid in 86% yield.1H-NMR (CDCl3/TMS, δ ppm): 3.97 (3H, s), 7.33 (1H, dd), 8.17 (1H, dd), 8.53 (1H, dd ).

67-56-1
790 suppliers
$7.29/5ml-f

2942-59-8
825 suppliers
$5.00/25g

40134-18-7
230 suppliers
$6.00/5g
Yield:40134-18-7 93%
Reaction Conditions:
with thionyl chloride for 8 h;Reflux;
Steps:
1.1 preparation of compound II
To the 250 ml flask is added in three 15.76g (0.1 µM) 2 - chloro -3 - picolinic acid, 100 ml methanol, under the ice-bath adds by drops 17.85g (0.15 µM) thionyl chloride, stirring and heating to reflux, the stirring reaction 8h. Thin layer chromatography tracking response, the raw materials point disappears, stopping the reaction. Reduced pressure distillation to remove the solvent to obtain light yellow 15.95g, yield 93%
References:
Qingdao University of Science and Technology;Wang Minghui;Yuan Jianpo;Huang Xuesong;Xu Liangzhong CN105061399, 2017, B Location in patent:Paragraph 0010; 0029; 0030

2942-59-8
825 suppliers
$5.00/25g

40134-18-7
230 suppliers
$6.00/5g

2942-59-8
825 suppliers
$5.00/25g

77-78-1
319 suppliers
$19.69/1ml

40134-18-7
230 suppliers
$6.00/5g

67-56-1
790 suppliers
$7.29/5ml-f
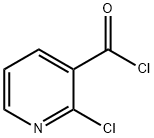
49609-84-9
252 suppliers
$20.00/5g

40134-18-7
230 suppliers
$6.00/5g

2942-59-8
825 suppliers
$5.00/25g

40134-18-7
230 suppliers
$6.00/5g