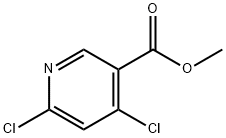
Methyl 4,6-dichloronicotinate synthesis
- Product Name:Methyl 4,6-dichloronicotinate
- CAS Number:65973-52-6
- Molecular formula:C7H5Cl2NO2
- Molecular Weight:206.03
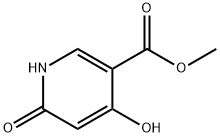
79398-27-9

65973-52-6
The general procedure for the synthesis of methyl 4,6-dichloronicotinate from methyl 4,6-dihydroxynicotinate was as follows: to a solution of methyl 4,6-dihydroxynicotinate (900 g, 5.3 mol) in phosphoryl chloride (4000 mL) was added N,N-diethylaniline (1035 mL, 6.4 mol). The reaction mixture was heated to 120 °C and maintained for 2 h, followed by cooling to room temperature. The reaction mixture was concentrated under vacuum and poured slowly into ice in batches. The mixture was extracted with ethyl acetate (2×). The organic extracts were combined, dried with anhydrous sodium sulfate and subsequently concentrated under vacuum. Purification of the crude product by rapid chromatography on silica gel (solvent gradient: 0% to 5% petroleum ether solution of ethyl acetate) afforded methyl 4,6-dichloronicotinate (400 g, 42% yield).LCMS (ESI) [M + H] = 206.1; 1H NMR (400 MHz, DMSO-d6) δ 8.84 (s, 1H), 7.98 (s, 1H) 3.90 (s, 3H).

79398-27-9
125 suppliers
$11.00/250mg

65973-52-6
244 suppliers
$6.00/1g
Yield:65973-52-6 97.2%
Reaction Conditions:
with triethylamine;trichlorophosphate at 0 - 120; for 2 h;
Steps:
1; 8 4,6-Dichloro-nicotinic acid methyl ester
4,6-Dihydroxy-nicotinic acid methyl ester (3.9 g, 23 mmol) (from Example 7 supra) was added to POCl3 (20 mL) at 0° C., then Et3N (5 mL) was added to the mixture slowly. After addition, the mixture was heated at 120° C. for 2 hours, then allowed to cool to 0° C. The reaction solution was poured slowly and portion-wise into ice-water (100 mL). Solid K2CO3 was added to adjust pH to 8. The resulting precipitate was collected by filtration. The solid was dissolved in ethyl acetate (50 mL), dried over anhydrous Na2SO4, filtered and concentrated to afford 4,6-dichloro-nicotinic acid methyl ester. (Yield 4.6 g, 97.2%). 1H NMR (301 MHz, CDCl3) δ 8.85 (s, 1H), 7.47 (s, 1H), 3.97 (s, 3H). LC-MS: [M+H]+ 205.9.
References:
US2012/184562,2012,A1 Location in patent:Page/Page column 6; 17
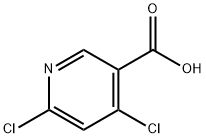
73027-79-9
267 suppliers
$13.00/1g

74-88-4
357 suppliers
$15.00/10g

65973-52-6
244 suppliers
$6.00/1g
93-60-7
474 suppliers
$8.00/25g

65973-52-6
244 suppliers
$6.00/1g

79398-27-9
125 suppliers
$11.00/250mg

65973-52-6
244 suppliers
$6.00/1g
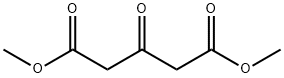
1830-54-2
409 suppliers
$9.00/10g

65973-52-6
244 suppliers
$6.00/1g