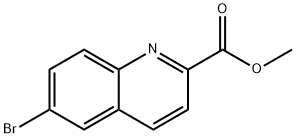
METHYL 6-BROMOQUINOLINE-2-CARBOXYLATE synthesis
- Product Name:METHYL 6-BROMOQUINOLINE-2-CARBOXYLATE
- CAS Number:623583-88-0
- Molecular formula:C11H8BrNO2
- Molecular Weight:266.09

67-56-1
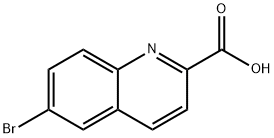
65148-10-9

623583-88-0
1c) Synthesis of methyl 6-bromo-2-quinolinecarboxylate A mixture of 6-bromo-2-quinolinecarboxylic acid (331 g, multiple batches, 1.31 mol) and methanesulfonic acid (22 mL, 33 g, 0.34 mol) in methanol (2 L) was refluxed for 6 hours. Upon completion of the reaction, the reaction mixture was neutralized with an aqueous solution of sodium bicarbonate (29 g, 0.34 mol) (350 mL). The resulting suspension was slowly cooled to 20 °C and stirred continuously overnight. The solid product was collected by filtration and the filter cake was washed with water (1 L). The solid product was dried in a vacuum oven at 50 °C for 3 days to give methyl 6-bromo-2-quinolinecarboxylate (294 g, 85% yield). 1H NMR (400 MHz, DMSO-d6) δ 8.52 (d, J = 9 Hz, 1H), 8.40 (d, J = 2 Hz, 1H), 8.14 (d, J = 9 Hz, 1H), 8.08 (d, J = 9 Hz, 1H), 7.97 (m, 1H), 3.93 (s, 3H). ES-LCMS m/z 267 (M + H)+.

67-56-1
790 suppliers
$7.29/5ml-f

65148-10-9
80 suppliers
$45.00/25mg

623583-88-0
48 suppliers
$125.00/100mg
Yield: 85%
Reaction Conditions:
Stage #1:methanol;6-bromo-quinoline-2-carboxylic acid with methanesulfonic acid for 6 h;Heating / reflux;
Stage #2: with water;sodium hydrogencarbonate in methanol at 20;
Steps:
1.1c
1c) Methyl 6-bromo-2-quinolinecarboxylate A mixture of 6-bromo-2-quinolinecarboxylic acid (331 g, from multiple batches, 1.31 mol) and methanesulfonic acid (22 mL, 33 g, 0.34 mol) in methanol (2 L) was refluxed for 6 hr. The mixture was treated with a solution of sodium bicarbonate (29 g, 0.34 mol) in water (350 mL) and the resulting suspension was slowly cooled to 20° C. and stirred overnight. The suspension was filtered and the cake was washed with water (1 L). The solid was dried in a vacuum oven at 50° C. for 3 days to yield methyl 6-bromo-2-quinolinecarboxylate (294 g, 85%). 1H NMR (400 MHz, DMSO-d6) δ 8.52 (d, J=9 Hz, 1H), 8.40 (d, J=2 Hz, 1H), 8.14 (d, J=9 Hz, 1H), 8.08 (d, J=9 Hz, 1H), 7.97 (m, 1H), 3.93 (s, 3H). ES-LCMS m/z 267 (M+H)+.
References:
SmithKline Beecham Corporation US2008/96921, 2008, A1 Location in patent:Page/Page column 32
511230-72-1
54 suppliers
$140.00/0.25 / G

623583-88-0
48 suppliers
$125.00/100mg
877-42-9
185 suppliers
$16.00/1g

623583-88-0
48 suppliers
$125.00/100mg
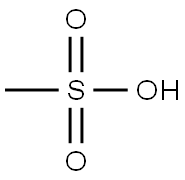
75-75-2
651 suppliers
$10.00/5g

65148-10-9
80 suppliers
$45.00/25mg

623583-88-0
48 suppliers
$125.00/100mg

106-40-1
484 suppliers
$12.00/5g

623583-88-0
48 suppliers
$125.00/100mg