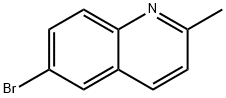
6-Bromo-2-methylquinoline synthesis
- Product Name:6-Bromo-2-methylquinoline
- CAS Number:877-42-9
- Molecular formula:C10H8BrN
- Molecular Weight:222.08

106-40-1

123-73-9

877-42-9
GENERAL METHOD: 4-Bromoaniline (1.50 mmol) and trans-2-butenal (1.00 mmol) were dissolved in 1.5 mL of ether (Et2O) in a reaction flask fitted with a magnetic stir bar, followed by the addition of silver(I)-exchanged montmorillonite K10 (0.50 g). After stirring for 5 min, the solvent was removed under vacuum to obtain a dry powder. The reaction mixture was heated at 120 °C for 3 hours. After the reaction mixture was cooled to room temperature, it was filtered through a short silica gel plug and the solid residue was washed well with dichloromethane (CH2Cl2). After removal of the solvent under vacuum, the crude product was purified by silica gel column chromatography, eluting using a solvent mixture of hexane/ethyl acetate (EtOAc) to give the final 6-bromo-2-methylquinoline (3a-3k). Note: For recycling/reuse studies, the crude product is separated by gravity filtration and the clay material is washed several times with dichloromethane (CH2Cl2), air-dried and weighed. The process is repeated for successive recycling/reuse reactions.
Yield:877-42-9 81%
Reaction Conditions:
with Ag(I) exchanged montmorillonite K10 in neat (no solvent) at 120; for 3 h;Green chemistry;Doebner-von Miller Reaction;
Steps:
General procedure for the synthesis of quinoline derivatives
General procedure: Amine (1.50 mmol) and aldehyde (1.00 mmol) were dissolved in 1.5 mL of Et2O in a reaction vial equipped with a magnetic stirrer bar, followed by the addition of Ag(I)-exchanged Montmorillonite K10 (0.50 g). After 5 min stirring, the solvent was removed in vacuo to obtain a dry powder. The reaction mixture was heated at a temperature of 120 oC for 3 h. After cooling to room temperature, the reaction mixture was filtered through a short silica plug and the solid residues washed well with CH2Cl2. The solvent was removed in vacuo and the crude product purified by column chromatography over silica gel eluting with a mixture of Hexane EtOAc to produce the title compounds (3a-3k). NOTE. For the recycle/reuse study, the crude product was gravity filtered and the clay material washed several times with CH2Cl2, air dried and weighed. This process was repeated for consecutive recycle/reuse reactions.
References:
Jayram, Janeeka;Jeena, Vineet [Heterocycles,2016,vol. 92,# 12,p. 2213 - 2224]
109-92-2
278 suppliers
$16.00/25mL

106-40-1
484 suppliers
$12.00/5g

877-42-9
185 suppliers
$16.00/1g

