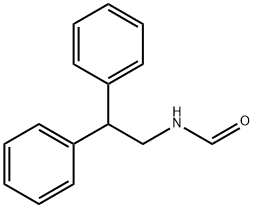
N-(2,2-Diphenylethyl)formamide synthesis
- Product Name:N-(2,2-Diphenylethyl)formamide
- CAS Number:19501-33-8
- Molecular formula:C15H15NO
- Molecular Weight:225.29
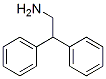
3963-62-0
151 suppliers
$10.00/1g
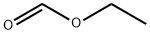
109-94-4
506 suppliers
$10.00/10g

19501-33-8
3 suppliers
$70.68/50mgs:
Yield:-
Reaction Conditions:
Reflux;
Steps:
1.1 Step 1)
Step 1)
Preparation of 2,2-Diphenylethylisocyanide.
A stirred suspension of 2,2-diphenylethylamine (7.00 g, 35.5 mmol) in ethyl formate (25 mL) was heated at reflux overnight.
The reaction was concentrated to afford the corresponding formamide as an off-white solid.
The crude product was taken up in methylene chloride (50 mL) and diisopropylamine (13.3 mL, 94.9 mmol) and cooled in an ice bath.
While stirring, phosphorus oxychloride (5.0 mL, 54 mmol) was added.
One hour later, the ice bath was removed and aqueous sodium carbonate solution (75 mL), water (50 mL) and methylene chloride (50 mL) were added.
The mixture was stirred vigorously for 1 hour.
The organic layer was washed with water, dried (sodium sulfate) and concentrated.
The resulting crude product was filtered through a plug of silica (methylene chloride) to afford 6.62 g (90%) of product as an off-white solid: 1H NMR (CDCl3) δ 7.38-7.18 (m, 10H), 4.35 (t, J= 7.5 Hz, 1H), 3.98 (d, J = 7.3, 2H) ppm.
References:
EP1294699,2016,B1 Location in patent:Paragraph 0066

64-18-6
1132 suppliers
$22.12/250ML

3963-62-0
151 suppliers
$10.00/1g

19501-33-8
3 suppliers
$70.68/50mgs:
107-31-3
355 suppliers
$16.00/25mL

3963-62-0
151 suppliers
$10.00/1g

19501-33-8
3 suppliers
$70.68/50mgs: