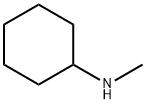
N-Methylcyclohexylamine synthesis
- Product Name:N-Methylcyclohexylamine
- CAS Number:100-60-7
- Molecular formula:C7H15N
- Molecular Weight:113.2

67-56-1
790 suppliers
$7.29/5ml-f
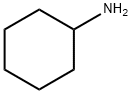
108-91-8
631 suppliers
$10.00/5mL

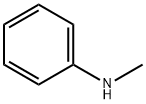
100-60-7
339 suppliers
$16.00/25mL
Yield:100-60-7 74 %Chromat.
Reaction Conditions:
with platinum on carbon;hydrogen;sodium hydroxide at 130; under 30003 Torr; for 36 h;Autoclave;
Steps:
2.4. Typical procedures of catalytic reactions
General procedure: After the reduction under a flow of H2 at 300°C for 0.5h, we carried out catalytic tests without exposing the catalyst to air as follows. Methanol (30mmol) was injected to the reduced catalyst inside the glass tube through a septum inlet, thus the catalyst was covered with a layer of methanol to restrict it from air exposure. After removal of the septum under air, amine (1mmol), solid NaOH (1mmol), n-dodecane (0.25mmol) and a magnetic stirrer bar were placed in the tube. The tube was inserted into a stainless-steel autoclave (28cm3) and purged with N2 gas. Finally, the resulting mixture was heated at 150°C and stirred under 1barN2. For the model reaction of n-octylamine, the catalyst screening, optimization of reaction conditions, kinetic studies and control reactions, the conversion of n-octylamine and yields of products were determined by GC analyses, using n-dodecane as an internal standard by applying the GC sensitivity of the isolated or commercial products. For the substrate scope study, the products were isolated by column chromatography with silica gel 60 (spherical, 60-100μm, Kanto Chemical Co., Ltd.) using hexane/ethyl acetate or ethyl acetate/methanol as the eluting solvent. The yields of the isolated amine derivatives were determined and identified by 1H and 13C NMR and GC-MS methods.
References:
Jamil, Md.A.R.;Touchy, Abeda S.;Rashed, Md. Nurnobi;Ting, Kah Wei;Siddiki, S.M.A. Hakim;Toyao, Takashi;Maeno, Zen;Shimizu, Ken-ichi [Journal of Catalysis,2019,vol. 371,p. 47 - 56]
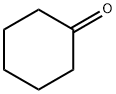
100-61-8
401 suppliers
$8.00/1g

100-60-7
339 suppliers
$16.00/25mL
108-94-1
584 suppliers
$12.00/50g

124-40-3
545 suppliers
$18.00/100ml

100-60-7
339 suppliers
$16.00/25mL
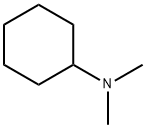
98-94-2
295 suppliers
$13.00/100g
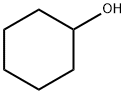
108-93-0
444 suppliers
$11.00/25g

67-56-1
790 suppliers
$7.29/5ml-f

108-91-8
631 suppliers
$10.00/5mL

100-60-7
339 suppliers
$16.00/25mL

98-94-2
295 suppliers
$13.00/100g
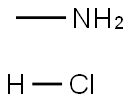
593-51-1
540 suppliers
$21.00/100g

108-94-1
584 suppliers
$12.00/50g

100-60-7
339 suppliers
$16.00/25mL