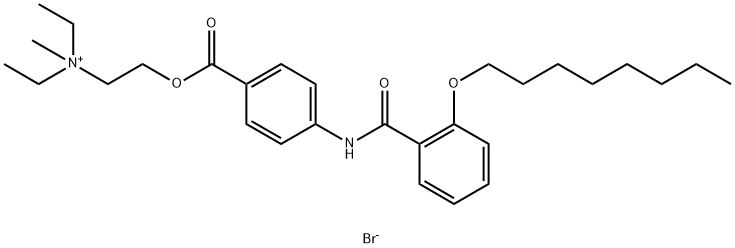
Otilonium bromide synthesis
- Product Name:Otilonium bromide
- CAS Number:26095-59-0
- Molecular formula:C29H43BrN2O4
- Molecular Weight:563.58
74-83-9
0 suppliers
$24.10/48624
![N-DIETHYLAMINOETHYL-P-[2-(-N-OCTYLOXY)-BENZOYL]AMINOBENZOATE](/StructureFile/ChemBookStructure6/GIF/CB6362586.gif)
26090-29-9
43 suppliers
$125.00/10mg

26095-59-0
210 suppliers
$5.00/50mg
Yield:26095-59-0 33%
Reaction Conditions:
in acetone at 60; for 48 h;Reflux;Sealed tube;Inert atmosphere;
Steps:
1.F [0120] General Procedure- Step F: alkylation to make quaternary amine salt (10).
A solution of 9 (100 mg, 0.21 mmol, 1 equiv) in acetone (2.5 ml) was added bromomethane (60 mg, 0.64 mmol, 3 equiv) and the resulting reaction was sealed and heated to reflux at 60°C for 2 days. The reaction was cooled to ambient temperature and the white precipitates were collected through filtration and washed with cold acetone to obtain the title compound 10 (40 mg, 33% yield) as white powder.1H NMR (400 MHz, DMSO-d6) d 10.45 (s, 1H), 7.97 (d, J = 8.0 Hz, 2H), 7.88 (d, J = 8.0 Hz, 2H), 7.62 (d, J = 7.6 Hz, 1H), 7.50 (dd, J = 8.0, 6.8 Hz, 1H), 7.18 (d, J = 8.4 Hz, 1H), 7.06 (dd, J = 7.6, 7.6 Hz, 1H), 4.66 (m, 2H), 4.10 (t, J = 6.4 Hz, 2H), 3.75 (m, 2H), 3.45 (q, J = 7.2 Hz, 4H), 3.07 (s, 3H), 1.74 (m, 2H), 1.38 (m, 2H), 1.28- 1.15 (m, 14H), 0.80 (t, J = 7.2 Hz, 3H). LCMS (m/z) 483.4 (M); RT (Method A, std LCMS method), 3.83 min.
References:
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM;DAVIES, Bryan;MCHARDY, Stanton;CUNNINGHAM, Ashley;WANG, Hua-Yu WO2020/163479, 2020, A1 Location in patent:Paragraph 0120
119-36-8
1049 suppliers
$5.00/10g

26095-59-0
210 suppliers
$5.00/50mg

111-83-1
499 suppliers
$12.00/25g

26095-59-0
210 suppliers
$5.00/50mg
100-37-8
13 suppliers
$14.00/25mL

26095-59-0
210 suppliers
$5.00/50mg
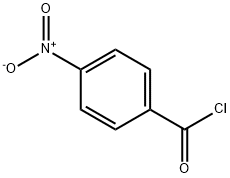
122-04-3
40 suppliers
$15.00/1g

26095-59-0
210 suppliers
$5.00/50mg