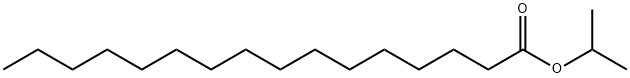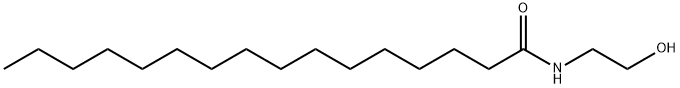
Palmitoylethanolamide synthesis
- Product Name:Palmitoylethanolamide
- CAS Number:544-31-0
- Molecular formula:C18H37NO2
- Molecular Weight:299.49

141-43-5
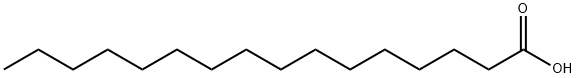
57-10-3

544-31-0
1) Under nitrogen protection, triethylamine (2 mL) was slowly added dropwise to a reaction vessel containing mercaptomethyl resin (2 g, MATRIX-INN), N-hydroxymaleimide (1.1 g, 9.7 mmol) and DMF (40 mL). After stirring at room temperature for 24 hours, the temperature was raised to 55°C and stirring was continued for 4 hours. The reaction mixture was cooled to room temperature and filtered, and the resulting NHS resin was washed twice each with DMF, distilled water and isopropanol sequentially, and dried under vacuum. 2) Palmitic acid (1.465 g, 5.72 mmol), NHS resin (1.50 g) prepared above, DIC (diisopropylcarbodiimide, 0.72 g, 5.72 mmol) and triethylamine (2 mL) were suspended in dichloromethane (15 mL). After stirring for 4 h at room temperature, the filtrate was filtered and retained for subsequent reactions (the amount of palmitic acid, DIC and solvent in the filtrate was detected by HPLC). The resin was washed twice each with DMF, water, isopropanol and dichloromethane sequentially, and dried under vacuum to obtain 1.70 g of dried resin with a loading of 1.0 mmol/g of immobilized palmitic acid active ester. 3) Ethanolamine (93.2 mg, 1.58 mmol) was added to a flask containing the activated ester (1.75 g) and 50 mL of ethanol, and suspended and stirred for 0.5 hr. The solid resin was removed by centrifugation and the resin was washed twice with ethanol (the resin was vacuum dried after washing). The liquid phases were combined and concentrated under reduced pressure to give 453 mg of palmitic acid monoethanolamide in 96.6% yield and >99.5% purity (HPLC detection).
Yield:544-31-0 98.2%
Reaction Conditions:
Stage #1: 1-hexadecylcarboxylic acid in water at 100; for 2 h;
Stage #2: ethanolamine in water at 100 - 200; for 22 h;
Steps:
Add 1280Kg of the obtained high-purity compound palmitic acid A into the reactor, add 1280Kg of deionized water, and under stirring, uniformly raise the temperature to 100°C within 1 hour, keep the temperature stable for 1 hour, and then gradually add the high-purity compound to the reactor. The compound palmitic acid A is 1.0-1.2 times, preferably 1.06 times the number of moles of monoethanolamine solution, control the dropping rate, add all the monoethanolamine solution dropwise within 2 hours, then increase the temperature and control the acylation reaction temperature at 150200 , preferably 155165, stir for 20 hours, after the acylation reaction is completed, keep it at 100115 for 0.5-1.5 hours, preferably 1 hour, remove the bottom water phase, and quickly remove the upper layer of the reaction liquid. Centrifugation and cooling until a flaky solid is precipitated and dried to obtain about 1470Kg of flaky hexadecyl amide ethanol (PEA);The compound A palmitic acid obtained from the hydrolysis reaction and rectification of this scheme,The purity can reach more than 99.5%. This makes the yield in the acylation reaction up to 98.2%, and the purity of the final product, hexadecyl amide ethanol (PEA), can reach more than 99%.
References:
CN112778119,2021,A Location in patent:Paragraph 0033