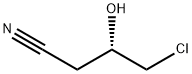
(S)-4-Chloro-3-hydroxybutyronitrile synthesis
- Product Name:(S)-4-Chloro-3-hydroxybutyronitrile
- CAS Number:127913-44-4
- Molecular formula:C4H6ClNO
- Molecular Weight:119.55
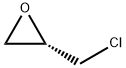
67843-74-7
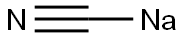
143-33-9

127913-44-4
1. 75 L of water was added to the reactor followed by (S)-epichlorohydrin (99.3% ee). Under stirring conditions, 4.76 kg of 25% aqueous sodium cyanide and 3.30 kg of 50% aqueous citric acid were slowly added dropwise for 1 h. 2. The pH was maintained between 7.9 and 8 during the reaction, and the temperature was controlled at 22-25°C for 50 min. 3. After completion of the reaction, stirring was continued for 10 h. Then 0.7 kg of sodium chloride was added and stirred until it was The reaction mixture was extracted with 20 L of ethyl acetate and the organic layer was separated. 5. 0.2 kg of anhydrous sodium sulfate was added to the ethyl acetate layer, stirred for 30 min and filtered. 6. The ethyl acetate was removed by evaporation under reduced pressure, and the residue was distilled through a membrane distiller at 110°C/1 mbar to give 1.77 kg of (S)-4-chloro-3-hydroxybutanenitrile (Yield 91.3%, chemical purity 99.1%). 7. and 99.1% chemical purity).7. The optical purity of the product was determined to be 99.3% ee by GC analysis.8. The experiments were repeated under different reaction conditions according to the same method and the results are summarized in Table 1.

67843-74-7
487 suppliers
$10.00/1g
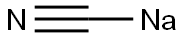
143-33-9
0 suppliers
$10.00/1g

127913-44-4
290 suppliers
$5.00/5g
Yield:127913-44-4 84.9%
Reaction Conditions:
with citric acid in water; pH=7.5 - 8.7 at 22 - 25; for 7 - 23 h;Product distribution / selectivity;Industry scale;
Steps:
1
3. 75 L of water was added to (S)-epichlorohydrin (99. 3% ee), and while stirring 4. 76 kg of 25% aqueous sodium cyanide solution and 3. 30 kg of 50% aqueous citric acid solution were dropped for 1 h 50 min to the solution under maintaining the reaction condition to pH 7. 9~8. 2 and 22~25C. After additional 10h stirring, 0. 7 kg of sodium chloride was added and dissolved. The resulting solution was extracted with 20 L of ethyl acetate. 0. 2 Kg of anhydrous sodium sulphate was added to the ethyl acetate layer, and then the solution was stirred for 30 min and filtered. The ethyl acetate was evaporated under reduced pressure, and the residue was distilled with thin film distillator (110 C/1 mbar) to give 1. 77 kg of the targeted compound (yield 91. 3%, chemical purity 99. 1%). Optical purity (GC) = 99. 3% ee In the same manner as described in the Experimental Example l, the reaction was performed under various conditions summarized in Table 1
References:
RSTECH CORPORATION WO2005/87715, 2005, A1 Location in patent:Page/Page column 9-10
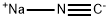
773837-37-9
0 suppliers
inquiry
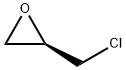
51594-55-9
376 suppliers
$10.00/1g

127913-44-4
290 suppliers
$5.00/5g

67843-74-7
487 suppliers
$10.00/1g
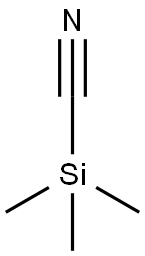
7677-24-9
394 suppliers
$19.00/5g

127913-44-4
290 suppliers
$5.00/5g
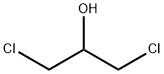
96-23-1
282 suppliers
$10.00/1g
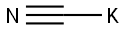
151-50-8
0 suppliers
$36.39/100g
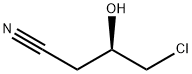
84367-31-7
223 suppliers
$6.00/1g

127913-44-4
290 suppliers
$5.00/5g
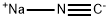
773837-37-9
0 suppliers
inquiry

106-89-8
779 suppliers
$9.00/10 g

84367-31-7
223 suppliers
$6.00/1g

127913-44-4
290 suppliers
$5.00/5g