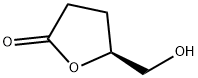
(S)-5-Hydroxymethyldihydrofuran-2-one synthesis
- Product Name:(S)-5-Hydroxymethyldihydrofuran-2-one
- CAS Number:32780-06-6
- Molecular formula:C5H8O3
- Molecular Weight:116.12

67-56-1
790 suppliers
$7.29/5ml-f
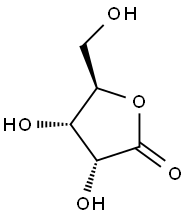
5336-08-3
202 suppliers
$10.00/100mg

32780-06-6
164 suppliers
$38.00/5g
196080-40-7
0 suppliers
inquiry
Yield:32780-06-6 75% ,196080-40-7 22%
Reaction Conditions:
with potassium perrhenate;phosphoric acid;palladium on activated charcoal;hydrogen;pyrographite at 150; under 3878.71 Torr;
Steps:
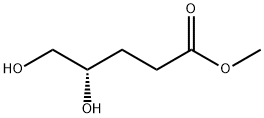
The table above demonstrates the significant yield improvements achieved by the addition of charcoal. The yield of adipate was improved to 91% from 70% by addition of activated charcoal, while the same ease of use (no atmosphere exchange, all reactions to the adipate carried out under the same set of conditions) was maintained. The catalyst system is also competent in the transformation of other diols in ctj3-position to carboxylate moieties as demonstrated in the table above. The final example in the table, i.e., 1,5-gluconolactone, demonstrates this selectivity within a single molecule: only the ctj3-diol group is transformed. The other hydroxy functionalities are not affected.[0136] A Parr reactor charged with polyol (7.5 mmol), KReO4 (22 mg), 10% Pd/C (60 mg), 85% H3P04 (26 mg), granular activated carbon (450 mg, C270C, purchased from Fisher), and MeOH (7.5 mL) was pressurized to 75 psi with H2. The reaction was placed in a preheated oil bath set to 150°C for a given amount of time until the reaction was complete (typically from a few hours up to three days). The reaction mixture was cooled to room temperature, filtered, rinsed with MeOH, and concentrated
References:
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA;BASF, SE;TOSTE, Dean, F.;LARSON, Reed, T.;BOHN, Martin, A. WO2017/147098, 2017, A1 Location in patent:Paragraph 0134; 0135; 0136
![(1S,5R)-6,8-dioxabicyclo[3.2.1]octan-4-one](/CAS/20200331/GIF/53716-82-8.gif)
53716-82-8
28 suppliers
inquiry

32780-06-6
164 suppliers
$38.00/5g
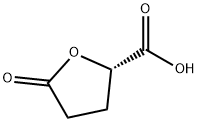
21461-84-7
200 suppliers
$19.00/250mg

32780-06-6
164 suppliers
$38.00/5g
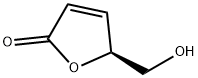
78508-96-0
80 suppliers
$47.39/250mg

32780-06-6
164 suppliers
$38.00/5g
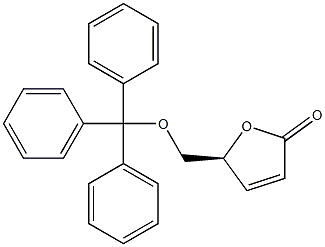
76236-32-3
2 suppliers
inquiry

32780-06-6
164 suppliers
$38.00/5g