
TRANS-2-NONENAL synthesis
- Product Name:TRANS-2-NONENAL
- CAS Number:18829-56-6
- Molecular formula:C9H16O
- Molecular Weight:140.22

111-66-0
173 suppliers
$10.00/10 mL
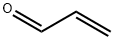
107-02-8
0 suppliers
$38.60/4s8501

18829-56-6
176 suppliers
$6.00/1g
Yield:18829-56-6 75%
Reaction Conditions:
with Hoveyda-Grubbs catalyst second generation in dichloromethane at 20; for 8 h;Schlenk technique;Inert atmosphere;
Steps:
8 2.3.4. Reactions with various substrates
General procedure: To a Schlenk flask loaded with catalyst 3 (13.4 mg, 2 mol%)were added dichloromethane (40 mL), decane (0.2 mL, 1 mmol)and either 1-decene, 1-octene, 1-hexene or 2-allyloxy-6-methylheptane (1 mmol) along with acrolein, crotonaldehyde,methacrolein or acrylonitrile (1 mmol). The reaction mixtureswere stirred, under nitrogen, for 24 h. Samples for GC analysiswere taken as described above (Section 2.3.2.1). In order to isolatethe cross-metathesis products the reactions were carried out inthe same way, in dichloromethane, but samples were not takenfor GC analysis. In each case, most of the solvent was removedunder vacuum after 24 h, and the remaining mixture passedthrough a silica column (5 cm × 2.5 cm) prepared with hexane.Hexane (20 mL) was flushed through the column followed bydiethyl ether:hexane (20:80; ca. 100 mL). Fractions containingthe desired product were identified by TLC, when eluting withthe diethyl ether:hexane mixture. These fractions were com-bined, and the solvents removed under vacuum. The remaining residue was then dried under vacuum overnight, yielding thecorresponding cross-metathesis product (see Table 3 for isolatedyields and Appendix A for NMR data of the products derived from2-allyloxy-6-methylheptane).
References:
Rountree, Sandra M.;Taylor, Sarah F.R.;Hardacre, Christopher;Lagunas, M. Cristina;Davey, Paul N. [Applied Catalysis A: General,2014,vol. 486,p. 94 - 104]
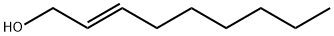
31502-14-4
78 suppliers
$49.00/10mL

18829-56-6
176 suppliers
$6.00/1g

111-66-0
173 suppliers
$10.00/10 mL

123-73-9
235 suppliers
$40.00/5g

18829-56-6
176 suppliers
$6.00/1g
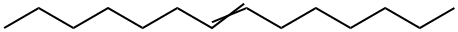
10374-74-0
48 suppliers
$91.00/5mL

111-25-1
321 suppliers
$10.00/5g

18829-56-6
176 suppliers
$6.00/1g
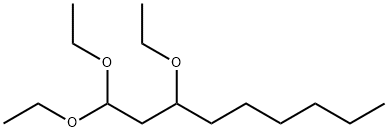
38872-36-5
0 suppliers
inquiry

18829-56-6
176 suppliers
$6.00/1g