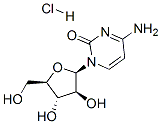
1-beta-D-Arabinofuranosylcytosine hydrochloride
- CAS No.
- 69-74-9
- Chemical Name:
- 1-beta-D-Arabinofuranosylcytosine hydrochloride
- Synonyms
- ARA-C;ac1075;ALEXAN;u-19920a;Cylocide;Ara-c.Ha;Cytarbine;ARA-C HCL;CYTARABINE HCL;ara-chydrochloride
- CBNumber:
- CB2260590
- Molecular Formula:
- C9H14ClN3O5
- Molecular Weight:
- 279.68
- MDL Number:
- MFCD00012839
- MOL File:
- 69-74-9.mol
- MSDS File:
- SDS
| Melting point | 197-198 °C(lit.) |
|---|---|
| storage temp. | 2-8°C |
| solubility | insoluble in EtOH; ≥14.2 mg/mL in DMSO; ≥64.2 mg/mL in H2O |
| form | crystalline |
| color | White to off-white |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | KCURWTAZOZXKSJ-XKIMMOKZSA-N |
| CAS DataBase Reference | 69-74-9(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | ARA-C |
| FDA UNII | 33K3DB6591 |
| EPA Substance Registry System | Cytarabine hydrochloride (69-74-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317-H319-H341-H361 | |||||||||
| Precautionary statements | P201-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 36-43-63 | |||||||||
| Safety Statements | 26-36/37 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | HA5500000 | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29389090 | |||||||||
| Toxicity | LD50 oral in rat: > 3200mg/kg | |||||||||
| NFPA 704 |
|
1-beta-D-Arabinofuranosylcytosine hydrochloride price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | C6645 | Cytosine β-D-arabinofuranoside hydrochloride crystalline | 69-74-9 | 1g | $400 | 2024-03-01 | Buy |
| Sigma-Aldrich | C6645 | Cytosine β-D-arabinofuranoside hydrochloride crystalline | 69-74-9 | 5g | $1400 | 2024-03-01 | Buy |
| Alfa Aesar | J65671 | Cytosine-beta-D-arabinofuranose hydrochloride | 69-74-9 | 1g | $50.65 | 2024-03-01 | Buy |
| Alfa Aesar | J65671 | Cytosine-beta-D-arabinofuranose hydrochloride | 69-74-9 | 5g | $95.65 | 2024-03-01 | Buy |
| Sigma-Aldrich | C6645 | Cytosine β-D-arabinofuranoside hydrochloride crystalline | 69-74-9 | 25mg | $51.8 | 2024-03-01 | Buy |
1-beta-D-Arabinofuranosylcytosine hydrochloride Chemical Properties,Uses,Production
Description
Cytosine beta-D-arabinofuranoside Hydrochloride is the salt form of Cytarabine, which is a selective inhibitor of DNA synthesis and is used as an antineoplastic and antiviral. Cytosine beta-D-arabinofuranoside Hydrochloride is used in chemotherapy for the treatment of white blood cells cancers such as acute myeloid leukemia (AML) and non-Hodgkin lymphoma.
Chemical Properties
Crystalline
Originator
Cytosar,Upjohn,US,1969
Uses
Antiviral.
Uses
Interferes with the synthesis of DNA
Manufacturing Process
(A) Preparation of 1- (2,3,5-Tri-O-Acetyl-β-D-Arabinofuranosyl)-4-Thiouracil: A
mixture of 1.85 g (5.0 mmol) of 1-(2,3,5-tri-O-acetyl-β-arabinofuranosyl)
uracil, 1.23 g (5.55 mmol) of phosphorus pentasulfide, and 30 ml of pyridine
was heated under gentle reflux for 2.5 hours with exclusion of moisture. The
reaction mixture was cooled, and the supernatant solution was transferred by
means of a pipette into a mixture of crushed ice and water. The reaction flask
was washed twice with pyridine, and these washings were added to the icewater mixture. This mixture was kept at about 25°C until the ice had melted,
and was then stored at 0°C for one hour. A pale yellow precipitate that formed
was collected on a filter, washed with ice-water, and dried in air.
This material was triturated with chloroform, and the chloroform mixture was
filtered. A small amount of undissolved material collected on the filter and it was washed with chloroform. The chloroform solution (filtrate plus washings)
was washed three times with ice-water, twice with ice-cold 3 N sulfuric acid,
twice with ice-cold saturated aqueous sodium bicarbonate solution, twice with
ice-water, and then dried over anhydrous sodium sulfate. The chloroform was
removed under reduced pressure at a bath temperature of about 40°C,
leaving a yellow, somewhat gummy residue. This yellow residue was dissolved
in absolute methanol which was then evaporated at reduced pressure at about
40°C, and the residue was then held for 2 hours at 0.5 to 2.0 mm pressure
and a bath temperature of about 50°C. There was thus obtained 1.69 g of 1-
(2,3,5-tri-O-acetyl-β-D-arabinofuranosyl)-4-thiouracil.
(B) Preparation of 1-β-D-Arabinofuranosylcytosine: In a glass liner, a mixture
of 1.16 g (3.0 mmol) of 1-(2,3,5-tri-O-acetyl-β-D-arabinofuranosyl)-4-
thiouracil prepared in (A) and about 60 ml of absolute methanol which had
been saturated with anhydrous ammonia at 0°C was heated in a steel bomb
at 98° to 105°C for 35 hours. After cooling to about 25°C and venting the
bomb, the dark solution was filtered into a round-bottom flask. The methanol
and excess ammonia were then removed under reduced pressure at about
25°C. The residual syrup was dissolved in absolute methanol, and the
methanol was removed under reduced pressure at a bath temperature of
about 40°C. This procedure of dissolving in absolute methanol and removing
the solvent was repeated, and the residue was held under reduced pressure at
a bath temperature of 45°C for 12 hours.
The resulting semisolid was triturated thoroughly with absolute methanol, and
the resulting suspension was chilled at 0°C. A pale tan solid that separated
was collected on a filter and washed repeatedly with methanol. After washing
with anhydrous ether, there was obtained 430 mg of 1-β-Darabinofuranosylcytosine.
(C) Preparation of 1-β-D-Arabinofuranosylcytosine Hydrochloride: The absolute
methanolic filtrate obtained after triturating and filtering the 1-β-Darabinofuranosylcytosine in (B) above was warmed and stirred with
decolorizing charcoal. The mixture was filtered through a bed of filter aid, and
the filter bed was washed repeatedly with absolute methanol. The combined
filtrate and washings were pale yellow. The solution was diluted to faint
cloudiness with anhydrous ether, and an excess of anhydrous hydrogen
chloride was introduced. Crystallization began at about 25°C and further
crystallization was induced by chilling at 0°C for 14 hours. The crystalline
product was collected on a filter, washed with anhydrous ether, and dried in
air. There was thus obtained 180 mg of pale yellow 1-β-Darabinofuranosylcytosine hydrochloride melting at 186° to 189°C.
The pale yellow product was dissolved in warm, absolute methanol, and the
solution after mixing with decolorizing charcoal was filtered through a bed of
filter aid. The filter bed was washed with warm absolute methanol, and the
combined methanolic filtrate and washings were warmed and diluted with
anhydrous ether to incipient crystallization. The methanol-ether mixture was
kept at about 25°C for about 1 hour and then chilled, first at 0°C, and then at
-20°C. The resulting colorless needles were collected on a filter, washed with
anhydrous ether, and dried at 85°C, yielding 100 mg of 1-β-Darabinofuranosylcytosine hydrochloride having a melting point of 186° to
188°C.
Therapeutic Function
Cancer chemotherapy
General Description
Crystals (from aqueous ethanol) or fluffy white powder.
Air & Water Reactions
Water soluble. Hydrolysis occurs readily, especially with acid.
Reactivity Profile
1-beta-D-Arabinofuranosylcytosine hydrochloride undergoes decomposition in acidic solutions.
Fire Hazard
Flash point data for 1-beta-D-Arabinofuranosylcytosine hydrochloride are not available; however, 1-beta-D-Arabinofuranosylcytosine hydrochloride is probably combustible.
Biological Activity
cytarabine hydrochloride is an effective drug in the treatment of cancers of white blood cells [1].cytarabine hydrochloride is a dxoxycytidine (dc) analogue. cytarabine hydrochloride has been found to be phosphorylated into a triphophate form, and thus compete with dctp for incorporation into dna. cytarabine hydrochloride has reported to incorporate into dna and block dna synthesis by inhibiting the function of dna and rna polymerases. in addition, incorporated has shown a growth inhibition dose-dependent curve using acute myelogenous leukemia (aml) in a growth inhibition assay with ic50 values of 16nm,103μm and 223μm for ccrf-cem, cem/arac8c and cem/dck-cell lines, respectively. moreover, cytarabine hydrochloride has been exhibited to retrovirally transducer rat leukemic ka cells by wst-1 assay with ic50 values of 0.69μm,1.73μm and 0.037μm for ka, ka/gfp and ka/wt, respectively [1,2].
Biochem/physiol Actions
Ara-C is phosphorylated to Ara-CTP and is incorporatee into DNA. It inhibits DNA replication by forming cleavage complexes with topoisomerase I resulting in DNA fragmentation, and ultimately induces apoptosis via the PKC signaling pathway. Does not inhibit RNA synthesis. Anti-leukemia agent.
Safety Profile
Poison by intravenous route.Moderately toxic by intraperitoneal and subcutaneousroutes. Experimental reproductive effects. A human eyeirritant. Mutation data reported. When heated todecomposition it emits very toxic fumes of NOx and HCl.
References
[1] tobias sc1, borch rf.synthesis and biological evaluation of a cytarabine phosphoramidate prodrug. mol pharm. 2004 mar-apr; 1(2):112-6.
[2] veuger mj1, heemskerk mh, honders mw, willemze r, barge rm.functional role of alternatively spliced deoxycytidine kinase in sensitivity to cytarabine of acute myeloid leukemic cells. blood. 2002 feb 15; 99(4):1373-80.
1-beta-D-Arabinofuranosylcytosine hydrochloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Beijing Yibai Biotechnology Co., Ltd | 0086-182-6772-3597 | sales04@yibaibiotech.com | CHINA | 419 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from 1-beta-D-Arabinofuranosylcytosine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-16 | 1-beta-D-Arabinofuranosylcytosine hydrochloride
69-74-9
|
US $0.00 / KG | 100g | 98%+ | 100kg | WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD | |
 |
2021-09-29 | 1-beta-D-Arabinofuranosylcytosine hydrochloride
69-74-9
|
US $0.00 / Kg/Bag | 1KG | 99%min HPLC | 100kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2021-07-13 | cytosine arabinoside hydrochloride
69-74-9
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- 1-beta-D-Arabinofuranosylcytosine hydrochloride
69-74-9
- US $0.00 / KG
- 98%+
- WUHAN CIRCLE POWDER TECHNOLOGY CO.,LTD
-

- 1-beta-D-Arabinofuranosylcytosine hydrochloride
69-74-9
- US $0.00 / Kg/Bag
- 99%min HPLC
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- cytosine arabinoside hydrochloride
69-74-9
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd