| Identification | More | [Name]
Ramelteon | [CAS]
196597-26-9 | [Synonyms]
RAMELTEON
rac N-[-[(8S)-1,6,7,8-Tetrahydro-2H-indeno[5,4-b]furan-8-yl]ethyl]propanamide
rac Ramelteon
rac Rozerem
TAK-375
RAMELTEON(FORR&DONLY)
(S)-N-[2-(1,6,7,8-Tetrahydro-2H-indeno-[5,4-b]furan-8-yl)ethyl]propionamide
N-[-[(8S)-1,6,7,8-Tetrahydro-2H-indeno[5,4-β]furan-8-yl]ethyl]propanamide
Ramelteon (approx. 90% S)
Rozerem
rac N-[-[(8S)-1,6,7,8-Tetrahydro-2H-indeno[5,4-β]furan-8-yl]ethyl]propanamide
N-[-[(8S)-1,6,7,8-Tetrahydro-2H-indeno[5,4-b]furan-8-yl]ethyl]propanamide | [EINECS(EC#)]
200-835-2 | [Molecular Formula]
C16H21NO2 | [MDL Number]
MFCD08067736 | [Molecular Weight]
259.34 | [MOL File]
196597-26-9.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Solid | [Melting point ]
113-1150C | [alpha ]
D20 -57.8° (c = 1.004 in chloroform) | [Boiling point ]
455.3±24.0 °C(Predicted) | [density ]
1.119±0.06 g/cm3(Predicted) | [Fp ]
2℃ | [storage temp. ]
Sealed in dry,Store in freezer, under -20°C | [solubility ]
Dimethyl Sulfoxide, Ethanol, Methanol, | [form ]
Solid | [pka]
16.37±0.46(Predicted) | [color ]
Crystalline | [Optical Rotation]
[α]/D -50 to -60°, c =1.0 in chloroform-d | [Usage]
Melatonin MT1/MT2 receptor agonist. Sedative, hypnotic | [InChI]
InChI=1S/C16H21NO2/c1-2-15(18)17-9-7-12-4-3-11-5-6-14-13(16(11)12)8-10-19-14/h5-6,12H,2-4,7-10H2,1H3,(H,17,18)/t12-/m0/s1 | [InChIKey]
YLXDSYKOBKBWJQ-LBPRGKRZSA-N | [SMILES]
C(NCC[C@H]1C2=C3CCOC3=CC=C2CC1)(=O)CC | [CAS DataBase Reference]
196597-26-9(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
Ramelteon, also known as N-[2-[(8S)-1,6,7,8-Tetrahydro-2H-indeno[5,4-b]furan-8-yl]ethyl]propanamide or 196597-26-9, is a melatonin agonist developed by Takeda Pharmaceuticals, Inc. It was approved by the FDA for marketing in the United States in September 2005 and is marketed under the name Rozerem. It is used to treat difficult-to-sleep and short-term insomnia. Ramelteon is effective for both chronic and short-term insomnia. Unlike most treatments of insomnia that target the GABA (g-aminobutyric acid) receptor complex, ramelteon is an agonist of the melatonin receptor. In particular, it has high selectivity for the MT1 and MT2 subtypes, which have been implicated in the maintenance of circadian rhythms, over the MT3 receptor responsible for other melatonin functions. Its lack of affinity for not only the GABA receptor complex but also neurotransmitter, dopaminerigic, opiate, and benzodiazepine receptors suggests an improved safety profile devoid of the abuse potential of the hypnotic drugs that target these receptors. As such, ramelteon is not a scheduled drug. | [Chemical Properties]
Crystalline Solid | [Originator]
Takeda (Japan) | [Uses]
Melatonin MT1/MT2 receptor agonist. Sedative, hypnotic | [Uses]
melatonin receptor agonist | [Definition]
ChEBI:N-[2-[(8S)-2,6,7,8-tetrahydro-1H-cyclopenta[e]benzofuran-8-yl]ethyl]propanamide is a member of indanes. | [Brand name]
Rozerem (Takeda). | [General Description]
The melatonin molecule wasmodified mainly by replacing the nitrogen of the indole ringwith a carbon to give an indane ring and by incorporating 5-methoxyl group in the indole ring into a more rigid furan ring.The selectivity of the resulting ramelteon for MT1 receptor iseight times more than that of MT2 receptor. Unlike melatonin,it is more effective in initiating sleep (MT1 activity)rather than to readjust the circadian rhythm (MT2 activity). Itappears to be distinctly more efficacious than melatonin butless efficacious than benzodiazepines as a hypnotic.Importantly, this drug has no addiction liability (it is not acontrolled substance). As a result, it has recently been approvedfor the treatment of insomnia. | [Synthesis]
Vilsmeier-Haack reaction on benzofuran 112 provided
aldehyde 113 (100%), which was converted to olefin 114
(88%) by Horner-Emmons reaction with triethylphosphonoacetate,
and was followed by hydrogenation of the olefin to
give ester 115 (100%). In order to avoid the cyclization of
the acid chloride intermediate into the wrong position, the
benzene ring was protected by bromination. Both bromination
and hydrolysis of the ester is accomplished in a single
pot to give acid 116. Thus the ester is brominated with bromine
in sodium acetate and acetic acid at 0??C and RT for several hours followed by quenching of remaining bromide
by sodium thiosulfate. The resulting acidic solution was
taken up in acetonitrile and refluxed for 2hr to provide the
acid 116 in 73% yield. The conversion of the acid to acid
chloride was done by reacting with thionyl chloride in odichlorobenzene
at 40??C for 30 to 40 min after which the
reaction was cooled to 0??C . Aluminum trichloride was
added and the reaction mixture was stirred at 0??C for 30 min
to deliver cyclized ketone 117 in 92% yield. After completion
of the cyclization, the bromines are removed by hydrogenation
(86%) and resulting ketone 118 was then reacted
under Horner-Emmons condition with diethyl cyano phosphonate
to give vinyl nitrile 119 in 84% yield. Selective reduction
of the nitrile was accomplished by hydrogenation
under basic condition (sodium hydroxide in toluene) in the presence of the activated cobalt at 25-50??C for 6.5 hr. The
amine was recovered as hydrochloride salt 120 (99% yield)
by treating the amine with HCl in methanol. In the next step,
the amine salt 120 was taken up in toluene and treated with
sodium hydroxide followed by hydrogenation of the mixture
with [RuCl(benzene)(R)-BINAP]Cl as catalyst to provide
chiral amine 121, after several work up and palladium catalyzed
hydrogenations, in 73% overall yield. Final acylation
of the amine with propionyl chloride in the presence of
aqueous sodium hydroxide in THF at room temperature gave
the desired product ramelteon (XVI), after crystallization, in
97% yield.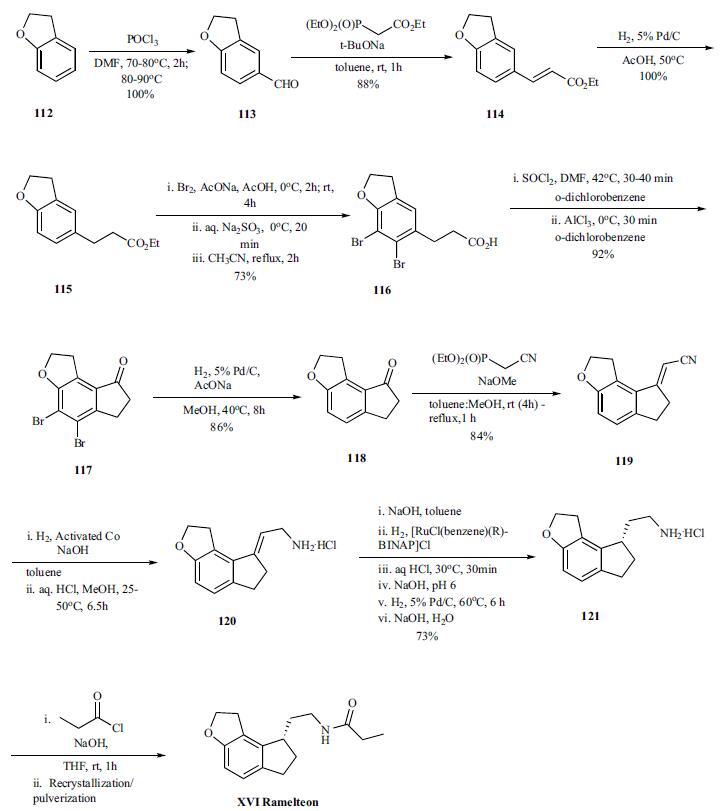
| [Drug interactions]
Since CYP1A2 is the major isozyme involved in the hepatic metabolism of ramelteon, it should not be taken in combination with strong CYP1A2 inhibitors, such as fluvoxamine. Co-administration with either ketoconazole (a CYP3A4 inhibitor) or fluconazole (a potent CYP2C9 inhibitor) resulted in significant increases in AUC and Cmax, but the extensive metabolism and highly variable plasma concentrations of ramelteon precluded the need for dose modification. The package insert, however, cautions patients about co-administration with potent CYP3A4 and CYP2C9 inhibitors. | [storage]
Store at -20°C | [References]
[1] miyamoto m. pharmacology of ramelteon, a selective mt1/mt2 receptor agonist: a novel therapeutic drug for sleep disorders. cns neurosci ther. 2009 winter;15(1):32-51. doi: 10.1111/j.1755-5949.2008.00066.x. |
|
|