| Identification | More | [Name]
Methyl 4-chloropicolinate | [CAS]
24484-93-3 | [Synonyms]
4-CHLORO-PYRIDINE-2-CARBOXYLIC ACID METHYL ESTER
IFLAB-BB F2108-0101
METHYL 4-CHLORO-2-PYRIDINECARBOXYLATE
METHYL 4-CHLOROPICOLINATE
METHYL 4-CHLOROPYRIDINE-2-CARBOXYLATE
Methyl 4-chloropyridine-2-carboxylate 97%
6-Methyl-2-(trifluoromethanesulfonyl)Oxypyridine
2-Pyridinecarboxylic acid, 4-chloro-, methyl ester | [EINECS(EC#)]
677-529-4 | [Molecular Formula]
C7H6ClNO2 | [MDL Number]
MFCD04116183 | [Molecular Weight]
171.58 | [MOL File]
24484-93-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Brown Solid | [Melting point ]
50-52 | [Boiling point ]
105-111 °C(Press: 2-3 Torr) | [density ]
1.294±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
-0.25±0.10(Predicted) | [color ]
Off-White to Beige | [InChI]
InChI=1S/C7H6ClNO2/c1-11-7(10)6-4-5(8)2-3-9-6/h2-4H,1H3 | [InChIKey]
VTENWIPSWAMPKI-UHFFFAOYSA-N | [SMILES]
C1(C(OC)=O)=NC=CC(Cl)=C1 | [CAS DataBase Reference]
24484-93-3(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Brown Solid | [Uses]
Methyl 4-Chloropicolinate (cas# 24484-93-3) is a compound useful in organic synthesis. | [Production Methods]
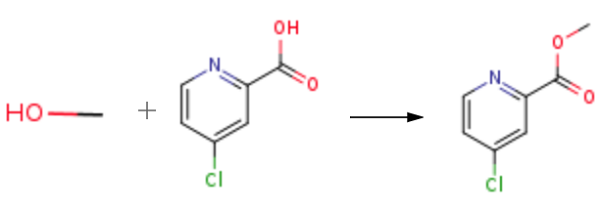
The starting material 2-picolinic acid was treated with SOCl2 to generate 4-chloropicolinoyl chloride, and then esterification with MeOH resulted in the formation of methyl 4-chloropicolinate. | [Synthesis Reference(s)]
Synthetic Communications, 26, p. 2017, 1996 DOI: 10.1080/00397919608003557 | [Synthesis]
To a suspension of 4-chloropyridine-2-carboxylic acid (4.5 g, 29.0 mmol) in methylene chloride (120 mL) was added oxalyl chloride (3.0 mL, 1.2 eq) under Ar2. The reaction was cooled to 0° C., and 500 uL of DMF was added. A large amount of gas was generated in situ. The reaction was stirred at room temperature for 1.5 h and then concentrated. Dry MeOH (50 mL) was added to the crude acyl chloride residue. The reaction was stirred at room temperature for 0.5 h, then quenched with NaHCO3 (5%) to neutral, extracted with EtOAc, and washed with brine. The combined organics were dried over MgSO4, filtered, and concentrated in vacuo to give 5.0 g of a crude solid, which was triturated with 5% EtOAc/hexane to give Methyl 4-chloropicolinate as a light yellow solid (4.5 g, 90%).
| [References]
[1] Patent: US2008/234332, 2008, A1. Location in patent: Page/Page column 22; 25
[2] Patent: CN105175325, 2017, B. Location in patent: Paragraph 0024; 0025
[3] Patent: WO2008/77188, 2008, A1. Location in patent: Page/Page column 28
[4] Patent: US9533973, 2017, B2. Location in patent: Page/Page column 34
[5] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 8, p. 2512 - 2515 |
|
|