| Identification | More | [Name]
Omeprazole | [CAS]
73590-58-6 | [Synonyms]
2-[(4-METHOXY-3,5-DIMETHYLPYRID-2-YL)-METHYLSULFO]-5-METHOXYBENZIMIDAZOLE
5-METHOXY-2-[[(4-METHOXY-3,5-DIMETHYL-2-PYRIDINYL)METHYL]SULFINYL]-1H-BENZIMIDAZOLE
5-METHOXY-2[(4-METHOXY-3,5-DIMETHYL-2-PYRIDYL)METHYLSULFINYL]-1H-BENZIMIDAZOLE
5-methoxy-2-((s)-((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-1h-benzimidazole
6-methoxy-2-[(r)-(4-methoxy-3,5-dimethyl-pyridin-2-yl)methylsulfinyl]-1h-benzoimidazole
ANTRA
ESOMEPRAZOLE
GASTROGARD
GASTROLOC
H 168/68
LOSEC
MEPRAL
MOPRAL
OMEPRAL
OMEPRAZOLE
PRILOSEC
R-(+)-OMEPRAZOLE
(S)-OMEPRAZOLE
ZOLTUM
1h-benzimidazole,5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulf | [EINECS(EC#)]
615-996-8 | [Molecular Formula]
C17H19N3O3S | [MDL Number]
MFCD00083192 | [Molecular Weight]
345.42 | [MOL File]
73590-58-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
156°C | [Boiling point ]
600.0±60.0 °C(Predicted) | [density ]
1.332 g/cm3 | [Fp ]
9℃ | [storage temp. ]
2-8°C
| [solubility ]
H2O: 0.5 mg/mL
| [form ]
solid
| [pka]
pKa 4.14/8.9(H2O,t =25,I=0.025) (Uncertain) | [color ]
white
| [Stability:]
Stable, but hygroscopic and photosensitive. Incompatible with strong oxidizing agents. Store in the dark. | [Water Solubility ]
Soluble in water (0.5 mg/ml), DMSO (25 mg/ml), and ethanol (4.5 mg/ml). | [Usage]
Binds covalently to proton pump. It inhibits gastric secretion. Used as an anttiulcerative | [Merck ]
6845 | [BCS Class]
2 | [Major Application]
forensics and toxicology
pharmaceutical (small molecule) | [InChI]
1S/C17H19N3O3S/c1-10-8-18-15(11(2)16(10)23-4)9-24(21)17-19-13-6-5-12(22-3)7-14(13)20-17/h5-8H,9H2,1-4H3,(H,19,20) | [InChIKey]
SUBDBMMJDZJVOS-UHFFFAOYSA-N | [SMILES]
COc1ccc2[nH]c(nc2c1)S(=O)Cc3ncc(C)c(OC)c3C | [CAS DataBase Reference]
73590-58-6(CAS DataBase Reference) | [EPA Substance Registry System]
1H-Benzimidazole, 6-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]- (73590-58-6) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
2
| [RTECS ]
DD9087000
| [HS Code ]
29333990 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Acute Tox. 4 Oral
Aquatic Chronic 2
Skin Sens. 1 | [Hazardous Substances Data]
73590-58-6(Hazardous Substances Data) | [Toxicity]
LD50 in mice, rats (g/kg): 0.08, >0.05 i.v.; >4, >4 orally (Ekman) |
| Hazard Information | Back Directory | [Description]
Omeprazole is a potent gastric antisecretory agent with selective inhibitory effect on the
H+,K+-ATPase proton pump. It is highly effective in the treatment of duodenal ulcer and
Zollinger-Ellison syndrome, and is reportedly superior to ranitidine in the management of
reflux esophagitis. | [Chemical Properties]
White Crystalline Solid | [Originator]
Astra (Sweden) | [History]
Omeprazole was first approved by the FDA in 1989 in the form of extended-release capsules for the treatment of acid-related disorders. Brand name Prilosec, it was initially used over-the-counter (OTC) for adults to help control heartburn that occurs two or more days a week. | [Uses]
Omeprazole is a proton pump inhibitor used to treat diseases like gastroesophageal reflux disease (GERD), used for gastric and duodenal ulcers, reflux or erosive esophagitis, and Zollinger-Ellison syndrome. It is also effective for gastric and duodenal ulcers that are ineffective with H2 receptor antagonists. Injections of Omeprazole can also be used for: 1 gastrointestinal bleeding, such as peptic and anastomic ulcer bleeding, and the prevention of severe diseases (such as cerebral hemorrhage, severe trauma, etc.) and gastric surgery caused by upper intestinal bleeding; 2 acute gastric mucosal damage complicated by stress or nonsteroidal anti-inflammatory drugs; 3 general anesthesia, post-surgery, or coma patients, to prevent acid reflux and aspiration pneumonia; 4 Combined with amoxicillin and clarithromycin, or with metronidazole and clarithromycin, it can effectively kill Helicobacter pylori (Hp). | [Application]
Omeprazole is a benzimidazole with selective and irreversible proton pump inhibition activity. Omeprazole forms a stable disulfide bond with the sulfhydryl group of the hydrogen-potassium (H+ - K+) ATPase found on the secretory surface of parietal cells, thereby inhibiting the final transport of hydrogen ions (via exchange with potassium ions) into the gastric lumen and suppressing gastric acid secretion. This agent exhibits no anticholinergic activities and does not antagonize histamine H2 receptors. Omeprazole Pellets are used in the treatment of Gastroesophageal reflux disease (GERD): A condition in which backward flow of acid from the stomach causes heartburn and injury of the food pipe (esophagus). | [Definition]
ChEBI: A member of the class of benzimidazoles that is 1H-benzimidazole which is substituted by a [4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl group at position 2 and a methoxy group at position 5. | [Preparation]
The antiulcer agent omeprazole is produced from 2,3,5-trimethylpyridine N-oxide.
Synthesis and Structure of Omeprazole
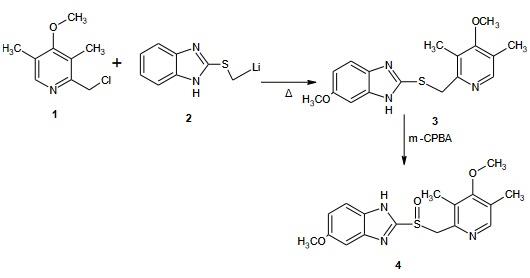
Steps: 2-(Lithium methyl sulphinyl)-5-methoxy-1H benzimidazole 20g was reacted with 2-chloro-3,5-dimethyl-4-methoxy pyridine 21 g to form sulphide intermediate and then converted to Omeprazole when treated with m-CPBA which used as anoxidizingagents. The acetamide-sulfide compounds modification are oxidised to form the amide sulfinyl compound and gives the sulfinyl carboxylate or salts upon alkaline hydrolysis.On further decarboxylation leads to the target molecules. The residual, unreacted salt, inorganic by-products and other minor by-products can be easily purified by a simple washing from omeprazole or lansoprazole. The amide compounds containing crystalline solids as opposed to the sulphide and sulfoxides of the reported procedures.
DOI: http://dx.doi.org/10.20902/IJPTR.2019.120307 | [Manufacturing Process]
3,5-Lutidine-N-Oxide
Hydrogen peroxide (45%, 200 ml) was added dropwise at 60°-70°C during 2 hours to a mixture of 3,5-Lutidine (125 g, 1.16 mole) and acetic acid (400 ml). The mixture was heated to 90°C and maintained at 90°-100°C for 2 hours after which it was cooled to 60°C. Again hydrogen peroxide (45%, 200 ml) was added dropwise at 60°-70°C during 1 hour and then the mixture was heated to 90°C and maintained at 90°-100°C for 6 hours. Thereafter, acetic acid and water was distilled off under reduced pressure and the distillation residue obtained was used as a starting product for the nitration.
3,5-Dimethyl-4-nitropyridine-N-oxide
To the distillation above obtained residue was added sulphuric acid (146 ml). Thereafter, a nitrating mixture consisting of sulphuric acid (250 ml) and nitric acid (280 ml) was added dropwise during 4 hours at 90°-100°C. The reactionmixture was heated further at 90°-100°C for 6 hours, after which it was cooled and poured over crushed ice (4 kg), Caustic lye (50%, 1150 ml) was added to the yellow solution and the precipitated crystalline compound was filtered under suction. The cake was washed with water and dried in vacuuo oven to yield the product which melted at 171°-173°C. Yield 78.5%. A sample crystallized from acetone had a melting point of 174°-174.5°C.
3,5-Dimethyl-4-nitropyridine-N-oxide-dimethyl sulfate adduct
To a suspension of 3,5-dimethyl-4-nitropyridine-N-oxide (150 g, 0.80 mole) in acetone (450 ml) was added dimethyl sulfate (90 ml, 0.95 mole). The mixture was heated to reflux until a clear solution was obtained and then allowed to cool to ambient temperature. An off-white crystalline solid separated out, which was filtered, washed with acetone and dried to yield 220 g of the adduct. Yield was 83.8% of theoretical.
3,5-Dimethyl-2-hydroxymethyl-4-nitropyridine
3,5-Dimethyl-4-nitropyridine-N-oxide-dimethyl sulfate adduct (220 g, 0.75 mole) was dissolved in methanol (1.0 ltr) and the solution heated to reflux. A solution of ammonium persulfate (140 gm) in water (200 ml) was added dropwise over 4 hours after which reflux was continued for 4 hours. Methanol was distilled off under reduced pressure and the residue was basified to pH 10 by addition of caustic lye (105 ml). The mixture was extracted with dichloromethane (2 times 400 ml). The dichloromethane layer was dried over sodium sulfate and filtered. The product was used as its solution in dichloromethane for the next reaction.
2-Chloromethyl-3,5-dimethyl-4-nitropyridine hydrochloride
To the cooled dichloromethane solution of 3,5-dimethyl-2-hydroxymethyl-4nitropyridine was added thionyl chloride (60 ml, 0.85 mole) dropwise over a period of 2 hours and stirring was continued for a further 2 hours. Methanol (10 ml) was added to destroy excess thionyl chloride and separated product was filtered under suction and washed with dichloromethane. The cake was dried in vacuum oven to yield 55 g of a cream colored product. Melting point was 124°-126°C.
5-Methoxy-2-[(3,5-dimethyl-4-nitro-2-pyridinyl)methylthio]-1H-benzimidazole
To a suspension of 5-methyl-2-mercaptobenzimidazole (36 g, 0.2 mole), 2chloromethyl-3,5-dimethyl-4-nitropyridine hydrochloride (47.4 g, 0.2 mole) and triethyl benzylammonium chloride (5 g) in a dichloromethane (500 ml) was added dropwise a solution of NaOH (17.6 gm, 0.44 mole) in water (30 ml). The addition was exothermic and the temperature was observed to rise to 40°C with reflux of dichloromethane - the reaction mixture was stirred for further 6 hours at ambient temperature and filtered. The cake was washed with water and dried in vacuum oven to yield 55.8 g of cream color product. Yield 81.1%; melting point 124°-128°C.
5-Methoxy-2-[(3,5-dimethyl-4-methoxy-2-pyridinyl)methylthio]-1Hbenzimidazole
5-Methoxy-2-[(3,5-dimethyl-4-nitro-2-pyridinyl)methylthio]-1Hbenzimidazole(50 g, 0.145 mole) was dissolved in methanol and heated to 45°C. A solution of sodium methoxide (50 g, 0.925 mole) in methanol (150 ml) was added dropwise over a period of 3 hours at 45°-60°C. Stirring was continued for another 2 hours and then methanol was distilled off under reduced pressure. To the cooled residue was added water (200 ml) followed by concentrated HCl (65 ml) until the pH of the mixture was 7.5. The reaction mixture was extracted with dichloromethane and the dichloromethane layer was washed with water (2 times 100 ml). The dichloromethane layer was dried over sodium sulfate and concentrated to yield the product as an amber color syrup. Yield was 40.1 gm, about 83.8% of theoretical. A solid sample was obtained by trituration of the syrup several times with petroleum ether. Melting point was 87°-90°C.
5-Methoxy-2-[(3,5-dimethyl-4-methoxy-2-pyridinyl)methylthio]-1Hbenzimidazolehydrochloride
HCl gas was bubbled into a cooled solution of 5-methoxy-2-[(3,5-dimethyl-4methoxy-2-pyridinyl)methylthiol]-1H-benzimidazole (50 g) in dichloromethane (250 ml) until no more precipitation was observed. The reaction mixture was warmed to 40°C and again cooled to 10°C. The solid was filtered under suction and washed with dichloromethane to yield the product (49 g) as a cream colored fine granular solid. Yield was 88.2% of theoretical. Melting point 144°-148°C.
Omeprazole from 5-methoxy-2-[(3,5-dimethyl-4-methoxy-2pyridinyl)methylthiol]-1H-benzimidazole
To a solution of 5-methoxy-2-[(3,5-dimethyl-4-methoxy-2pyridinyl)methylthio]-1H-benzimidazole (32.9 g, 0.1 mole) in dichloromethane (200 ml) was added phthalic anhydride (20 g, 0.135 mole) and cooled in an ice salt bath. This was followed by addition of sodium carbonate (18 g, 0.17 mole) and water (20 ml). Hydrogen peroxide (12 ml, 45%, 0.16 mm mole) was added dropwise at -5°-0°C and the reaction mixture was stirred at the same temperature. When the reaction was complete as indicated by TLC, water (200 ml) was added, cooling bath was removed and the reaction mixture was stirred for 10 mins. The organic layer was separated and washed with 5% sodium carbonate solution. The separated dichloromethane solution was charcoalised and filtered through celite. The filtrate was concentrated to 100 ml and ethyl acetate 100 ml was added thereto. The separated solid was filtered, washed with ethyl acetate and dried in vacuum oven to yield 28.20 g of omeprazole. Yield 82.4% of theoretical. Melting point was 158°-160°C (dec.).
| [Brand name]
. Prilosec (AstraZeneca). | [Therapeutic Function]
Antiulcer | [World Health Organization (WHO)]
Omeprazole was introduced in the 1980s. It belongs to a group of
agents that have an inhibitory effect on the secretion of hydrochloric acid in the
stomach (gastric acid proton pump inhibitors) and is used in the treatment of
upper gastrointestinal tract disorders. The Committee for Proprietary Medicinal
Products of the European Commission has concluded that a causal association
between the reactions reported in Germany and the use of omeprazole had not
been established. Nevertheless oral administration should be preferred.
(Reference: (CPMPPO) Pharmacovigilance Opinion, No.16 , , 25 July 1994) | [General Description]
Omeprazole, 5-methoxy-2-(((4-methoxy-3, 5-dimethyl-2-pyridinyl)methyl) sulfinyl)-1Hbenzimidazole(Losec), is a white to off-white crystallinepowder with very slight solubility in water. Omeprazole isan amphoteric compound (pyridine N, pKa 4.06; benzimidazoleN-H, pKa 0.79), and consistent with the proposedmechanism of action of the substituted benzimidazoles, isacid labile. Hence, the omeprazole product is formulatedas delayed-release capsules containing enteric-coatedgranules.
The absolute bioavailability of orally administeredorneprazole is 30% to 40% related to substantial first-passbiotransformation. The drug has a plasmahalf-life of about 1 hour. Most (77%) of an oral dose ofomeprazole is excreted in the urine as metabolites with insignificantantisecretory activity. The primary metabolitesof omeprazole are 5-hydroxyomeprazole (CYP2C19) andomeprazole sulfone (CYP3A4). The antisecretory actions ofomeprazole persist for 24 to 72 hours, long after the drughas disappeared from plasma, which is consistent with itssuggested mechanism of action involving irreversible inhibitionof the proton pump.
Omeprazole is approved for the treatment of heartburn,GERD, duodenal ulcer, erosive esophagitis, gastric ulcer,and pathological hypersecretory conditions. | [Biological Activity]
H + ,K + -ATPase inhibitor (IC 50 = 5.8 μ M) that displays antisecretory and antiulcer activity. Inhibits gastric acid secretion (IC 50 = 0.16 μ M for histamine-induced acid formation) and reduces gastric lesion formation induced by a variety of ulcerative stimuli. Antibacteral against Helicobacter pylori in vitro . Also inhibits CYP2C19, CYP2C9 and CYP3A (K i values are 3.1, 40.1 and 84.4 μ M respectively) and blocks swelling-dependent chloride channels (ICIswell). | [Biochem/physiol Actions]
Omeprazole binds covalently to proton pump (H+, K+-ATPase) and inhibits gastric secretion. It is useful in ameliorating the effects of peptic oesophagitis, duodenal and gastric ulcer. Omeprazole is preferred over antagonists of histamine H2-receptor and ranitidine for its higher efficiency. It is also useful in treating Zollinger-Ellison syndrome. | [Clinical Use]
Gastric acid suppression | [Side effects]
The aforementioned adverse
events published as case reports seem to be extremely
rare relative to the large number of treatment
courses with omeprazole of well above
100 million. Some of these adverse effects, however,
disappeared upon cessation of omeprazole
therapy and reappeared upon rechallenge
suggesting a causal relationship. These include
cases of myopathy , neuromyositis , erythema
nodosum , arthralgia , pityriasis
rosea-like skin eruption , hemolytic anemia
, interstitial nephritis , , CNSsymptoms
, , and acute gout . | [Veterinary Drugs and Treatments]
Omeprazole is potentially useful in treating both gastroduodenal
ulcer disease and to prevent or treat gastric erosions caused by
ulcerogenic drugs (e.g., aspirin). An oral paste product is labeled
for the treatment and prevention of recurrence of gastric ulcers in
horses. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: effect of coumarins possibly
enhanced.
Antiepileptics: effects of phenytoin possibly
enhanced.
Antifungals: absorption of itraconazole and
ketoconazole reduced; avoid with posaconazole;
concentration increased by voriconazole.
Antivirals: reduced atazanavir concentration
- avoid; AUC of saquinavir increased by 82%
(increased risk of toxicity) - avoid; concentration of
raltegravir possibly increased - avoid; concentration
of rilpivirine reduced - avoid; concentration of
omeprazole reduced by tipranavir.
Ciclosporin: variable response; mostly increase in
ciclosporin level.
Cilostazol: increased cilostazol concentration -
reduce cilostazol dose.
Clopidogrel: avoid due to reduced efficacy of
clopidogrel.
Cytotoxics: possibly reduced excretion of
methotrexate; avoid with erlotinib and vandetanib;
possibly reduced dasatinib and lapatinib absorption
- avoid with dasatinib; possibly reduced absorption
of pazopanib.
Tacrolimus: may increase tacrolimus concentration.
Ulipristal: reduced contraceptive effect, avoid with
high dose ulipristal. | [Metabolic pathway]
When male humans are given 14C-omeprazole orally,
an average of 79% of the dose is recovered in the
urine in 96 h. Omeprazole is completely metabolized
and at least six metabolites are identified. Two major
metabolites are hydroxyomeprazole and omeprazole
acid. | [Metabolism]
Omeprazole is completely metabolised in the liver by the
cytochrome P450 system to form inactive metabolites
which are excreted mostly in the urine and to a lesser
extent in bile. CYP2C19 produces hydroxyomeprazole,
the major metabolite, CYP3A4 produces omeprazole
sulphone. | [Mode of action]
Omeprazole is a proton pump inhibitor which can specifically act on gastric parietal cell proton pump sites and transform into the active form of sulfonamide, then irreversibly binds to the proton pumps through disulfide bonds, generating a sulfonamide and proton pump compound (H + -K + -ATP), thereby inhibiting the enzymatic activity, preventing the H+ in parietal cells from being transported to the stomach cavity. It has a strong and persistent inhibitory role on gastric acid secretion caused by basal gastric acid and pentapeptide gastric acid secretions, greatly reducing gastric acid within the gastric juice. Rapid, reversible, and no H2 antagonist-induced psychiatric side effects. | [References]
1) Satoh?et al.?(1989),?Antisecretory and antiulcer activities of a novel proton pump inhibitor AG-1749 in dogs and rats; J. Pharmacol. Exp. Ther.,?248?806
2) Kuzin?et al.?(2018),?Effects of the Proton Pump Inhibitors Omeprazole and Pantoprazole on the Cytochrome P450-Mediated Metabolism of Venlafaxine; Clin. Pharmacokinet,?57?729
3) Schmarda?et al.?(2000)?The gastric H,K-ATPase blocker Iansoprazole is an inhibitor of chloride channels; Br. J. Pharmacol.,?129?598
4) Maejima?et al.?(2020),?Oral oxytocin delivery with proton pump inhibitor pretreatment decreases food intake; Peptides,?128?170312
5) Wantanabe?et al.?(2020),?Selective Targeting of Virus Replication by Proton Pump Inhibitors; Sci. Rep.,?10?4003
6) Bojkova?et al.?(2020),?SARS-CoV2 and SARS-CoV differ in their cell tropism and drug sensitivity profiles;?bioRxiv, epub ahead of print?DOI: 10.1101/2020.04.03.024257 |
|
|