| Identification | More | [Name]
Enoxacin | [CAS]
74011-58-8 | [Synonyms]
1-ETHYL-6-FLUORO-1,4-DIHYDRO-4-OXO-7-[1-PIPERAZINYL]-1,8-NAPHTHYRIDINE-3-CARBOXYLIC ACID
ENOXACIN
1,8-naphthyridine-3-carboxylicacid,1,4-dihydro-1-ethyl-6-fluoro-4-oxo-7-(1-pi
1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthyridine-3-car
at-2266
bactidan
ci919
comprecin
enoram
Enoxacine
flumark
pd107779
Enoxacin (Patent-No-Supply)
ENX
Flumeork
Gyramid
Enoxacin (DMF)
1-ETHYL-6-FLUORO-1,4-DIHYDRO-4-OXO-7-(1-PIPERAZINYL)-1,8-NAPHTHYRIDINE-3-CARBOXYLIC ACID (ENOXACIN)
Abenox
Enoxen | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C15H17FN4O3 | [MDL Number]
MFCD00133308 | [Molecular Weight]
320.32 | [MOL File]
74011-58-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
220-224 C | [Boiling point ]
569.9±50.0 °C(Predicted) | [density ]
1.2931 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
6.04±0.70(Predicted) | [color ]
Off-White to Pale Yellow | [Water Solubility ]
50 mg/ml in 1 M NaOHSlightly soluble in sodium hydroxide, dimethyl sulfoxide, chloroform and methanol. Insoluble in water. | [InChI]
InChI=1S/C15H17FN4O3/c1-2-19-8-10(15(22)23)12(21)9-7-11(16)14(18-13(9)19)20-5-3-17-4-6-20/h7-8,17H,2-6H2,1H3,(H,22,23) | [InChIKey]
IDYZIJYBMGIQMJ-UHFFFAOYSA-N | [SMILES]
N1(CC)C2C(=CC(F)=C(N3CCNCC3)N=2)C(=O)C(C(O)=O)=C1 | [CAS DataBase Reference]
74011-58-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves . | [WGK Germany ]
2
| [RTECS ]
QN2800000
| [HS Code ]
29335990 | [Toxicity]
LD50 in male, female mice, male, female rats (mg/kg): 327, 391, 236, 294 i.v.; 1237, 1320, >2000, >2000 s.c.; all >5000 orally (Senda) |
| Hazard Information | Back Directory | [Description]
Enoxacin is a broad spectrum, quinolone-class, antibacterial agent closely related
structurally to nalidixic acid. The serum half-life (6.2 hrs.) and urinary recovery
(70%) are considerably greater than for other newer agents of this class, such as
norfloxacin (10) and earlier mentioned ciprofloxacin. | [Chemical Properties]
Off-White to Pale Yellow Solid | [Originator]
Dainippon (Japan) | [Uses]
A fluororquinolone antibacterial used to treat urinary tract infections and gonorrhea. | [Uses]
A flurorquinolone antibacterial used to treat urinary tract infections and gonorrhea. | [Definition]
ChEBI: A 1,8-naphthyridine derivative that is 1,4-dihydro-1,8-naphthyridine with an ethyl group at the 1 position, a carboxy group at the 3-position, an oxo sustituent at the 4-position, a fluoro substituent at the 5-position and a piperazin-1-yl group at the 7 p
sition. An antibacterial, it is used in the treatment of urinary-tract infections and gonorrhoea. | [Manufacturing Process]
2,6-Dichloro-3-nitropyridine was reacted with N-ethoxycarbonylpiperazine to
give 6-chloro-2-(4-ethoxycarbonyl-1-piperazinyl)-3-nitropyridine. The product,
without purification, was heated with ethanolic ammonia in a sealed tube at
120°-125°C to give 6-amino-2-(4-ethoxycarbonyl-1-piperazinyl)-3-
nitropyridine (mp 132°-134°C), which was treated with acetic anhydride in
acetic acid to give 6-acetylamino-2-(4-ethoxycarbonyl-1-piperazinyl)-3-
nitropyridine (mp 168°-169°C). This compound was catalytically hydrogenated
in the presence of 5% palladium-carbon in acetic acid to yield 3-amino-6-
acetylamino-2-(4-ethoxycarbonyl-1-piperazinyl)pyridine. The obtained 3-
amino derivative, without further purification, was dissolved in a mixture of
ethanol and 42% tetrafluoroboric acid, and to this solution was added a
solution of isoamyl nitrite in ethanol at below 0°C with stirring 20 minutes
later, ether was added to the solution. The resulting precipitate was collected
by filtration and washed with a mixture of methanol and ether and then with
chloroform to yield 6-acetylamino-2-(4-ethoxycarbonyl-1-piperazinyl)-3-
pyridine diazonium tetrafluoroborate; mp 117°-117.5°C (dec.).
A suspension of the diazonium salt in toluene was gradually heated and kept
at 120°C (bath temp.) for 30 minutes with stirring. After evaporation of the
solvent under reduced pressure, the residue was made alkaline with 10%
sodium carbonate and then extracted with chloroform. The chloroform extract
was dried over anhydrous potassium carbonate. After evaporation of the
solvent, the crystalline residue was recrystallized from ethyl acetate to give 6-
acetylamino-2-(4-ethoxycarbonyl-1-piperazinyl)-3-fluoropyridine (mp 132°-
133°C). The 3-fluoro derivative was hydrolyzed with a mixture of 15%
hydrochloric acid and methanol (1:2 v/v) to give 6-amino-2-(4-
ethoxycarbonyl-1-piperazinyl)-3-fluoropyridine. This compound was treated
with diethyl ethoxymethylenemalonate at 130°-140°C to give N-[2-(4-
ethoxycarbonyl-1-piperazinyl)-3-fluoro-6-pyridinyl]aminomethylenemalonate
(mp 144°-145°C) and then the product was cyclized by heating at 255°C to
give ethyl 7-(4-ethoxycarbonyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-
naphthyridine-3-carboxylate (mp 279°-281°C).
Preparation of 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-
naphthyridine-3-carboxylic acid and acid addition salts thereof.
Ethyl 7-(4-ethoxycarbonyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-
naphthyridine-3-carboxylate was suspended in dimethylformamide (10 ml)
and to the suspension was added potassium carbonate (0.53 g). After the
mixture was kept at 60°C for 10 minutes with stirring, ethyl iodide (1.2 g)
was added to the solution. The mixture was stirred for 2 hours at 60°-70°C.
The reaction mixture was concentrated to dryness under reduced pressure,
and water was added to the residue. After extraction with chloroform, the
chloroform extract was dried over anhydrous potassium carbonate. After
removal of the chloroform by distillation, the resulting precipitate was
recrystallized from a mixture of dichloromethane and n-hexane to give 0.89 g
of ethyl 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethoxycarbonyl-1-
piperazinyl)-1,8-naphthyridine-3-carboxylate (mp 171°-173°C). A mixture of
the above ethyl ester (0.8 g), 10% sodium hydroxide (6 ml) and ethanol (2
ml) was refluxed by heating for 3 hours. After cooling, the solution was
adjusted to pH 7.0-7.5 with 10% acetic acid. The precipitate was collected by
filtration, washed with ethanol and recrystallized from a mixture of
dimethylformamide and ethanol to give 0.57 g of 1-ethyl-6-fluoro-1,4-
dihydro-4-oxo-7-(1-piperazinyl)-1,8-naphthyridine-3-carboxylic acid. Melting
point 220°-224°C.
The above prepared carboxylic acid (0.2 g) thus obtained was dissolved in 5%
hydrochloric acid, and the solution was concentrated to dryness under reduced
pressure. The residue was crystallized from water to give a hydrochloride of
the (0.21 g), m.p. above 300°C. On the other hand, the above free carboxylic
acid (0.2 g) was dissolved in 7% methanesulfonic acid solution under heating.
After cooling, the precipitate was recrystallized from diluted methanol to give
a methanesulfonic acid salt of the carboxylic acid (0.22 g), mp above 300°C
(dec.).
The free carboxylic acid (1.0 g) was heated to dissolve in ethanol and then to
the solution was added acetic acid (1.0 ml). After the mixture was cooled, the
resulting crystals were collected and recrystallized from ethanol to give acetic
acid salt of the carboxylic acid (0.93 g), mp 228°-229°C. | [Brand name]
Penetrex (Sanofi Aventis);FLUMARK. | [Therapeutic Function]
Antibacterial, Antiinfective | [Pharmaceutical Applications]
A 1,8 naphthyridone derivative available as an oral drug. It exhibits good activity in vitro against many species of Enterobacteriaceae. It is inactive against Ps. aeruginosa, Serratia, Citrobacter, Acinetobacter and Mycobacterium spp., as well as anaerobes, chlamydiae and ureaplasmas.
It is well absorbed and widely distributed when given orally. Absorption is not significantly affected by food, but ranitidine, sucralfate and some antacids or mineral supplements may interfere with absorption. After repeated doses of 400 mg every 12 h for 14 days, mean peak plasma levels reach 3.5–4.5 mg/L, a steady state being achieved in 3–4 days. | [Clinical Use]
1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8- naphthyridine-3-carboxylic acid (Penetrex) is a quinolone with broad-spectrum antibacterial activity that is used primarily for the treatment of urinary tract infections and sexually transmitted diseases. Enoxacin has been approved for the treatment of uncomplicated gonococcal urethritis and has also been shown to be effective in chancroid caused by Haemophilus ducreyi. A single 400-mg dose is used for these indications. Enoxacin is also approved for the treatment of acute (uncomplicated) and chronic (complicated) urinary tract infections. Enoxacin is well absorbed following oral administration. Oral bioavailability approaches 98%. Concentrations of the drug in the kidneys, prostate, cervix, fallopian tubes, and myometrium typically exceed those in the plasma. More than 50% of the unchanged drug is excreted in the urine. Metabolism, largely catalyzed by cytochrome P450 enzymes in the liver, accounts for 15% to 20% of the orally administered dose of enoxacin. The relatively short elimination half-life of enoxacin dictates twice-a-day dosing for the treatment of urinary tract infections. Some cytochrome P450 isozymes, such as CYP 1A2, are inhibited by enoxacin, resulting in potentially important interactions with other drugs. For example, enoxacin has been reported to decrease theophylline clearance, causing increased plasma levels and increased toxicity. Enoxacin forms insoluble chelates with divalent metal ions present in antacids and hematinics, which reduce its oral bioavailability. | [Synthesis]
Enoxacin, 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8-naph�thiridin-3-carboxylic acid (33.2.24), only differs from norfloxacin in that the carbon atom
in position 8 of norfloxacin is replaced with a nitrogen atom, i.e. this drug belongs to the
napthiridine series and not the quinolone series. It is synthesized from 2,6-dichloro-3-
nitropyridine, which is reacted with N-ethoxycarbonylpiperazine, and which leads to sub�stitution of the chlorine atom at the second position of the pyridine ring to give (33.2.20).
Subsequent replacement of the chlorine atom at position C6 with an amino group (using
ammonia), acylation of the resulting amino group with acetic anhydride, and finally, reduc�tion of the nitro group at position C3 of the pyridine ring with hydrogen gives 6-amino-3-
acetylamino-2-(4-ethoxycarbonylpiperazinyl)pyridine (33.2.21). In order to introduce a
fluorine atom at position C3, a Schiemann reaction is carried out. To do this, the free amino
group is diazotated with amyl nitrite, and the resulting diazonium salt is treated with tetra�fluoroboric acid. The resulting diazonium tetrafluoroborate undergoes pyrolysis to give 3-
fluoro-6-acetylamino-2-(4-ethoxycarbonylpiperazinyl)pyridine. Finally, removing the acetyl protection of the amino group at position C6 gives 3-fluoro-6-amino-2-(4-ethoxycar�bonylpiperazinyl)pyridine (33.2.22). The resulting amine (33.2.22) is reacted with
ethyl ethoxymethylenmalonate, which results in the formation of a derivative of
aminomethylenmalonic ester, which upon heating gives the ethyl ester of 6-fluoro-1,4-
dihydro-4-oxo-7-(4-ethoxycarbonyl-1-piperazinyl)-1,8-naphthiridin-3-carboxylic acid
(33.2.23). Alkylating this with ethyl iodide followed by hydrolysis of the two carboethoxy
groups gives enoxacin.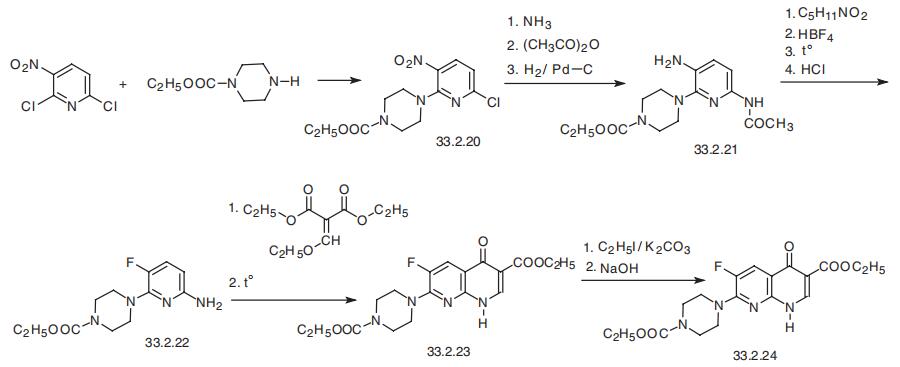
| [storage]
4°C, protect from light | [References]
[1] A M CLARKE M E C S J Zemcov. In-vitro activity of pefloxacin compared to enoxacin, norfloxacin, gentamicin and new beta-lactams.[J]. Journal of Antimicrobial Chemotherapy, 1985, 15 1: 39-44. DOI: 10.1093/jac/15.1.39
[2] M. TAKEI. Target Preference of 15 Quinolones against Staphylococcus aureus, Based on Antibacterial Activities and Target Inhibition[J]. Antimicrobial Agents and Chemotherapy, 2001, 139 1: 3544-3547. DOI: 10.1128/aac.45.12.3544-3547.2001
[3] M. OZAKI. In Vivo Evaluation of NM441, a New Thiazeto-Quinoline Derivative[J]. Antimicrobial Agents and Chemotherapy, 1992, 36 1: 1170-1170. DOI: 10.1128/aac.36.5.1170-b
[4] XUWEN LUO. Enoxacin inhibits proliferation and invasion of human osteosarcoma cells and reduces bone tumour volume in a murine xenograft model.[J]. Oncology Letters, 2020, 20 2: 1400-1408. DOI: 10.3892/ol.2020.11656 |
|
|