| Identification | More | [Name]
TRIMETHYLAMINE HYDROCHLORIDE | [CAS]
75-50-3 | [Synonyms]
FEMA 3241
N, N-DIMETHYLMETHYLAMINE HCL
TRIMETHYLAMINE HCL
TRIMETHYLAMINE HYDROCHLORIDE
TRIMETHYLAMMONIUM CHLORIDE
ai3-15639
dimethylmethaneamine
femanumber:3241
Methanamine, N,N-dimethyl-
Methanamine,N,N-dimethyl-
Methylamine, N,N-dimethyl-
n,n-dimethyl-methanamin
trimethylamine(solutions)
trimethylamine,anhydrous
trimethylamineanhydre
Trimethylaminesolution
trimethylaminesolutions
Trimethylamine water solution 33%
Trimethylamine anhydrate
Trimethylamine,water solution | [EINECS(EC#)]
209-810-0 | [Molecular Formula]
C3H10ClN | [MDL Number]
MFCD00012478 | [Molecular Weight]
95.57 | [MOL File]
75-50-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Trimethylamine is compressed gas or liquid.
Flammable gas. Shipped as a compressed gas, it may be
present in an aqueous solution. It has a strong, fishy, ammoniacal
odor. The Odor Threshold is 0.00011-0.87 ppm.
Warning: The Odor Threshold range is so broad that odor
alone should not be used as a warning of potentially
hazardous exposures. | [Melting point ]
-117 °C (lit.) | [Boiling point ]
3-4 °C (lit.) | [density ]
0.83-0.88 g/mL at 20 °C
| [vapor density ]
2.09 (vs air)
| [vapor pressure ]
430 mm Hg ( 25 °C)
| [FEMA ]
3241 | [refractive index ]
n20/D 1.357
| [Fp ]
38 °F
| [storage temp. ]
2-8°C
| [solubility ]
very soluble in water, slightly soluble in alcohol, ether, benzene, toluene, xylene, ethylbenzene, chloroform maximum allowable concentration: TLV 10 p.p.m. (24 mg/m3) and STEL of 15 p.p.m. (36 mg/m3) (ACGIH 1986) | [form ]
Liquid | [pka]
pKb (25°): 4.13 | [color ]
Colorless | [Odor]
smelling like rotting fish, rotting eggs, garbage, or urine. | [PH]
a strong base (pH 9.8) | [Stability:]
Stable. Incompatible with a wide variety of materials, including bases, acids, oxidizing agents, brass, zinc, magnesium, aluminium, mercury, mercury oxides, acid chlorides, acid anhydrides. Hygroscopic. Highly flammable. Readily forms explosive mixtures with air. | [biological source]
synthetic | [explosive limit]
11.6% | [Odor Threshold]
0.000032ppm | [Odor Type]
fishy | [Water Solubility ]
Soluble In Water, 8.9e+005 mg/L. | [FreezingPoint ]
-117.1℃ | [Sensitive ]
Hygroscopic | [JECFA Number]
1610 | [Merck ]
14,9710 | [BRN ]
956566 | [Dielectric constant]
2.9(4℃) | [Exposure limits]
ACGIH: TWA 50 ppm; STEL 100 ppm (Skin)
OSHA: TWA 200 ppm(590 mg/m3)
NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) | [InChI]
1S/C3H9N/c1-4(2)3/h1-3H3 | [InChIKey]
GETQZCLCWQTVFV-UHFFFAOYSA-N | [SMILES]
CN(C)C | [LogP]
0.06 | [Uses]
Organic synthesis, especially of choline salts,
warning agent for natural gas, manufacture of disinfectants,
flotation agent, insect attractant, quaternary
ammonium compounds, plastics. | [CAS DataBase Reference]
75-50-3(CAS DataBase Reference) | [EPA Substance Registry System]
Trimethylamine (75-50-3) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,C,F+,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R34:Causes burns.
R20/22:Harmful by inhalation and if swallowed .
R12:Extremely Flammable.
R41:Risk of serious damage to eyes.
R37/38:Irritating to respiratory system and skin .
R20:Harmful by inhalation. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S16:Keep away from sources of ignition-No smoking .
S29:Do not empty into drains . | [OEB]
A | [OEL]
TWA: 10 ppm (24 mg/m3), STEL: 15 ppm (36 mg/m3) | [RIDADR ]
UN 2924 3/PG 2
| [WGK Germany ]
1
| [RTECS ]
YH2700000
| [F ]
3-10 | [Autoignition Temperature]
374 °F | [TSCA ]
Yes | [DOT Classification]
2.1 (Flammable gas) | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29211100 | [Storage Class]
2A - Gases | [Hazard Classifications]
Acute Tox. 4 Inhalation
Eye Dam. 1
Flam. Gas 1A
Press. Gas Liquefied gas
Skin Irrit. 2
STOT SE 3 | [Safety Profile]
Poison by intravenous
route. Moderately toxic by subcutaneous
and rectal routes. Mildly toxic by inhalation.
A very dangerous fire hazard when exposed
to heat or flame. Self-reactive. Moderately
explosive in the form of vapor when
exposed to heat or flame. Can react with
oxidizing materials. To fight fire, stop flow
of gas. Potentially explosive reaction with
bromine + heat, ethylene oxide,
triethynylaluminum. When heated to
decomposition it emits toxic fumes of NOx.
See also AMINES. | [Hazardous Substances Data]
75-50-3(Hazardous Substances Data) |
| Hazard Information | Back Directory | [General Description]
A colorless gas with a fishlike odor at low concentrations changing to ammonia-like odor at higher concentrations. Shipped as a liquid under its own vapor pressure. Contact with the unconfined liquid can cause frostbite from evaporative cooling or chemical type burns. The gasis corrosive and dissolves in water to form flammable, corrosive solutions. Gas is an asphyxiate by the displacement of air. Produces toxic oxides of nitrogen during combustion. Prolonged exposure to heat can cause the containers to rupture violently and rocket. Long-term inhalation of low concentrations or short-term inhalation of high concentrations has adverse health effects. | [Reactivity Profile]
TRIMETHYLAMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. Contamination of an ethylene oxide tank with trimethylamine caused an explosion [BCISC Quart. Safety Summ., 1966, 37, 44]. | [Air & Water Reactions]
Highly flammable and easily ignited. Water soluble. | [Health Hazard]
VAPOR: POISONOUS IF INHALED. Irritating to eyes, nose, and throat. LIQUID: Will burn skin and eyes. Harmful if swallowed. | [Potential Exposure]
Trimethylamine is used as a chemical
intermediate in organic synthesis of quaternary ammonium
com pounds; as an insect attractant; as a warning agent in
natural gas; flotation agent. | [Fire Hazard]
FLAMMABLE. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area. Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Medical observation is recommended for 24-48 hours after
breathing overexposure, as pulmonary edema may be
delayed. As first aid for pulmonary edema, a doctor or
authorized paramedic may consider administering a drug or
other inhalation therapy. | [Shipping]
UN1083 Trimethylamine, anhydrous, Hazard
Class: 2.1; Labels: 2.1-Flammable gas. Cylinders must be
transported in a secure upright position, in a wellventilated
truck. Protect cylinder and labels from physical
damage. The owner of the compressed gas cylinder is the
only entity allowed by federal law (49CFR) to transport
and refill them. It is a violation of transportation regulations
to refill compressed gas cylinders without the express
written permission of the owner. UN1297 Trimethylamine,
aqueous solutions with not >50% trimethylamine by mass,
Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive
material. | [Incompatibilities]
A medium strong base. Violent reaction
with strong oxidizers (such as chlorine, bromine, fluorine),
ethylene oxide; nitrosating agents, for example, nitrites,
sodium nitrite, nitrous gases, nitrous acid) capable of
releasing carcinogenic nitrosamines.); keep away from mercury,
strong acids. Corrosive to many metals, for example,
zinc, brass, aluminum, copper, tin, and their alloys. | [Chemical Properties]
Trimethylamine is compressed gas or liquid.
Flammable gas. Shipped as a compressed gas, it may be
present in an aqueous solution. It has a strong, fishy, ammoniacal
odor. The Odor Threshold is 0.00011-0.87 ppm.
Warning: The Odor Threshold range is so broad that odor
alone should not be used as a warning of potentially
hazardous exposures. | [Waste Disposal]
Return refillable compressed
gas cylinders to supplier. Nonrefillable cylinders should be
disposed of in accordance with local, state and federal regulations.
Allow remaining gas to vent slowly into atmosphere
in an unconfined area or exhaust hood. Refillabletype
cylinders should be returned to original supplier with
any valve caps and outlet plugs secured and valve protection
caps in place. | [Physical properties]
Trimethylamine has a pungent, fishy, ammoniacal odor at low concentration.It's a colourless liquid with a boiling point around 3.5°C, compared with the higher melting point of 224-226°C for the more polar Me3NO, which presumably has dipole-dipole intermolecular forces.
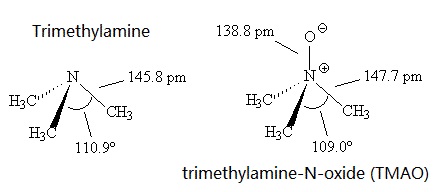
Trimethylamine is a base, like ammonia. Also like ammonia, it has a trigonal pyramidal structure. The C-N-C bond angle is 110.9°, compared with 107.2° in NH3, presumably due to greater repulsions between the methyl groups. This angle is reduced to 109.0° in Me3NO.
| [Occurrence]
TMA is widely distributed in the environment as a normal constituent of animal and plant tissue and as a result of its formation during the decay of organic matter in plants, animals, fish, sewage and animal waste (Graedel 1978; Hippe et al 1977; Oremland et al 1982). The amine is formed primarily as the result of microbial degradation of the plant and animal constituents betaine and choline and from bacterial reduction of trimethylamine oxide, a common constituent of aquatic organisms. It also occurs naturally in a variety of foodstuffs and in tobacco smoke and these are the most likely sources of human exposure (HSDB 1988).
Numerous strains of bacteria isolated from various sources have been found capable of growing on TMA (HSDB 1988). Degradation products formed under anaerobic conditions include dimethylamine, formaldehyde, formate and C02, while under aerobic conditions, TMA is converted to dimethylamine, ammonia and methane. | [Definition]
ChEBI: A tertiary amine that is ammonia in which each hydrogen atom is substituted by an methyl group. | [Preparation]
Trimethylamine can be synthesized from paraformaldehyde and ammonium chloride, by the reaction of formic acid, formaldehyde, and ammonia, and by interaction of methanol and ammonia with a catalyst at high temperature. | [Production Methods]
Trimethylamine (TMA) is produced by several methods: from the reaction of ammonia and methanol; from paraformaldehyde and ammonium chloride; by the action of formaldehyde and formic acid on ammonia; and by the interaction of methanol and ammonia over a catalyst at high temperature (Hawley 1981; HSDB 1988). TMA is sold as an aqueous solution or as a liquefied gas (Windholz et al 1983) in which the aqueous solution is available as 25, 30, and 40% and anhydrous as 99% minimum. The impurities consist of ammonia at no more than 0.2% by weight of solution and formaldehyde at no more than 0.3% by wt. of solution (Rick 1985). U.S. production was estimated to be approximately 15,322 tons in 1984 (HSDB 1988). | [Reactions]
Trimethylamine (TMA) has been used in the preparation of poly[9,9′-bis(6′-N,N,N-trimethylammonium)hexyl)fluorene-co-alt-4,7-(2,1,3-benzothiadiazole) dibromide] (PFBT), a water-soluble, cationic conjugated polymer used in label-free DNA microarrays. It can also be used to prepare benzyltrimethylammonium chloride, which then reacts with sodium ethoxide to form benzyltrimethylammonium ethoxide.The adsorption of TMA on the gold surface of trimethylsilylated barium nitrate-gold/titanosilicate catalyst acts as a promoter for the propylene epoxidation with oxygen and hydrogen. | [Aroma threshold values]
Detection: 0.3 to 0.8 ppb; recognition: 500 ppb | [Industrial uses]
TMA is used in the manufacturing of quaternary ammonium compounds, insect repellents, disinfectants, flotation agents, plastics, as a warning agent in gases, animal feed supplement, as a food additive for seafood flavor, and an ingredient in synthetic fermented egg protein product which is used to attract coyotes. In addition, it is used in organic synthesis of cationic emulsion polymers and as a chemical initiator for acetylcholine bromide [neuroregulator] (HSDB 1988; Anonymous 1981). The primary use (89%) is as a chemical intermediate for choline chloride (animal feed supplement), while 11% is used in other applications (HSDB 1988). | [Carcinogenicity]
No studies were found that examined the carcinogenicity of Trimethylamine in humans. Because mechanisms have been proposed by which the known carcinogen N47 nitrosodimethylamine can be formed from Trimethylamine and TMAO (Bain 2005) in the presence of nitrosating agents, there is some concern about the neoplastic potential of Trimethylamine. Thus, the German exposure guidelines warn that co-exposure to Trimethylamine and nitrosating agents should be minimized (see Section 8.2.). However, a 2-year mouse and rat inhalation study with the related amine DMA, which can also potentially form N-nitrosodimethylamine, showed no tumor formation despite severe chronic nasal lesions (CIIT 1990). | [Metabolism]
Aliphatic amines are well absorbed from the gut and respiratory tract (Beard and Noe 1981). However, TMA is a normal constituent of mammalian urine, complicating its pharmacokinetic analysis. TMA is partially metabolized to ammonia and subsequently to urea in mammals. It is converted in part to TMA oxide which is readily reduced back to TMA (Anon. 1972). TMA also is partially metabolized by N-demethylation to form formaldehyde (Parke 1968).
In an experiment using 4 male volunteers, over 95% of the administered TMA was excreted in the N-oxide form, confirming N-oxidation as the major route of metabolism in man (Al-Waiz et al 1987a). A condition known as trimethylaminuria (fish-odor syndrome) is likely to result from an inborn error in N-oxidation (Al-Waiz et al 1987b). Renal tubular transport and metabolism was investigated in chickens and TMA was found to be almost entirely metabolized in vivo to TMA oxide (Acara et al 1977).
In healthy humans, 2 mmol choline chloride, choline stearate, or lecithin administered orally markedly increased the urinary excretion of TMA, dimethylamine, and monomethylamine, with choline chloride having the greatest effect. Choline is known to be converted to TMA in mammals by gut microorganisms (HSDB 1988). When rats were treated with 1 mmol/kg of choline chloride or lecithin, urinary excretion of TMA was significantly increased while dimethylamine and monomethylamine excretion was not altered (Zeisel et al 1983).
TMA is reported to stimulate NADPH oxidation by an amount equivalent to the amount of TMA oxide formed (LaDu et al 1971). | [Purification Methods]
Dry triethylamine by passing the gas through a tower filled with solid KOH. Water and impurities containing labile hydrogen were removed by treatment with freshly sublimed, ground, P2O5. It has been refluxed with acetic anhydride, and then distilled through a tube packed with HgO and BaO. [Comyns J Chem Soc 1557 1955.] For more extensive purification, trimethylamine is converted to the hydrochloride, crystallised (see below), and regenerated by treating the hydrochloride with excess aqueous 50% KOH, the gas is passed through a CaSO4 column into a steel cylinder containing sodium ribbon. After 1-2 days, the cylinder is cooled to -78o and hydrogen and air are removed by pumping. [Day & Felsing J Am Chem Soc 72 1698 1950.] Me3N has been distlled from trap-to-trap and degassed by freeze-pump-thaw [Halpern et al. J Am Chem Soc 108 3907 1986]. It is commercially supplied in a pressure tin. [Beilstein 4 H 43, 4 I 322, 4 II 553, 4 III 99, 4 IV 134.] | [Toxics Screening Level]
The initial threshold screening level (ITSL)for trimethylamine is 120 μg/m3 based on an eight hour averaging time. |
|
|