- Fluorine nitrogen gas
-

- $0.00 / 20kg
-
2023-11-07
- CAS:7727-37-9
- Min. Order: 20kg
- Purity: 50%
- Supply Ability: 20mt
Related articles - What is nitrogen used for?
- Nitrogen is the most common pure element in the earth, frequently a by-product of air-processing for industrial concentration ....
- May 24,2024
- Fun facts about nitrogen
- Nitrogen is extremely important to living material. Plants, animals and humans could not live without it. In its liquid form, ....
- Mar 11,2024
|
| | Nitrogen Chemical Properties |
| Melting point | −210 °C(lit.) | | Boiling point | −196 °C(lit.) | | density | 1.2506 | | vapor density | 0.97 (vs air) | | solubility | At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 62 volumes of water and about 10 volumes of ethanol (96 per cent). | | form | colorless gas | | color | colorless | | Odor | odorless, tasteless | | Water Solubility | slightly soluble H2O; insoluble alcohol [HAW93] | | Merck | 13,6634 | | Dielectric constant | 1.0(20℃) | | Cosmetics Ingredients Functions | PROPELLANT | | InChI | 1S/N2/c1-2 | | InChIKey | IJGRMHOSHXDMSA-UHFFFAOYSA-N | | SMILES | N#N | | Surface tension | 5.13 mN/m at 95K,0.540MPa | | CAS DataBase Reference | 7727-37-9(CAS DataBase Reference) | | NIST Chemistry Reference | Nitrogen(7727-37-9) | | EPA Substance Registry System | Nitrogen (7727-37-9) |
| Safety Statements | 38 | | RIDADR | UN 1066 2.2 | | WGK Germany | - | | RTECS | QW9700000 | | F | 4.5-31 | | TSCA | TSCA listed | | DOT Classification | 2.2 (Nonflammable gas) | | HazardClass | 2.2 | | Storage Class | 2A - Gases | | Hazard Classifications | Press. Gas Compr. Gas | | Hazardous Substances Data | 7727-37-9(Hazardous Substances Data) |
| | Nitrogen Usage And Synthesis |
| History | Nitrogen was discovered independently in 1772 by Swedish chemist Carl Scheele and Scottish botanist Daniel Rutherford. Priestly, Cavendish, and Lavoisier also obtained nitrogen independently more or less around the same time. Nitrogen was recognized first as an element by Lavoisier, who named it “azote”, meaning “without life.” The element was named nitrogen in 1790 by Chaptal. The name derived from the Greek name ‘nitre’ for potassium nitrate which contains nitrogen.
| | Occurance | Nitrogen is the principal component of air. The earth’s atmosphere constitutes about 78% nitrogen by volume. Nitrogen also occurs as nitrates in several minerals such as Chile saltpeter (sodium nitrate), niter or saltpeter (potassium nitrate) and minerals containing ammonium salts. Nitrogen is contained in many complex organic molecules including proteins and amino acids that occur in all living organisms.
| | Uses | Gaseous nitrogen has numerous uses in chemical, food, metal, and electrical industries. Nitrogen is needed in commercial production of ammonia (Haber process) and in preparation of many nitrides. It also is the starting material in making cyanamide salts, cyanides, and nitrogen oxides for producing nitric acid. Other applications are in gas chromatrography, as a carrier gas, to provide an inert atmosphere in chemical reactions, to prevent oxidation reactions, to reduce fire or explosion hazards, and to dilute a reacting gas.
In the food industry nitrogen is used to prevent mold growth, spoilage from oxidation, and insect infestation.
Other miscellaneous applications of nitrogen gas include pressurizing cable jackets, preventing carburization in welding and soldering, inflating balloons, agitating liquid baths, and cooling catalytic reactors in petroleum refining.
| | Uses | Nitrogen has many commercial and technical applications. As a gas, it is used in heat treating of primary metals; blanketing of oxygen- sensitive liquids and of volatile liquid chemicals; the production of semiconductor electronic components, as a blanketing atmosphere; the blowing of foam-type plastics; the deaeration of oxygen-sensitive liquids; the degassing of nonferrous metals; food processing and packing; inhibition of aerobic bacteria growth; magnesium reduction of aluminum scrap; and the propulsion of liquids through pipelines.
Gaseous nitrogen is also used in pressurizing aircraft tires and emergency bottles to operate landing gear; purging, in the brazing of copper tubing for air-conditioning and refrigeration systems; the purging and filling of electronic devices; the purging, filling, and testing of high-voltage compression cables; the purging and testing of pipelines and related instruments; and the treatment of alkyd resins in the paint industry.
Liquid nitrogen also has a great many uses, among them the freezing of highly perishable foods such as shrimp, hamburgers, and chicken; deflashing of rubber tires; cooling of concrete; and the cold-trapping of materials such as carbon dioxide from gas streams (commonly used in this way in systems that produce high vacuums). It is used as a coolant for electronic equipment, for pulverizing plastics, and for simulating the conditions of outer space. Other ways in which liquid nitrogen is used include: creating a very high pressure gaseous nitrogen (15 000 psig or 103 000 kPa) through liquid nitrogen pumping; in food and chemical pulverization; for the freezing of liquids in pipelines for emergency repairs; for low temperature stabilization and hardening of metals; for low temperature research; for low temperature stress relieving of aluminum alloys; for the preservation of whole blood, livestock sperm, and other biologicals; for refrigerating foods in local and long-distance hauling; for refrigeration shielding of liquid hydrogen, helium, and neon; for the removal of skin blemishes in dermatology; and for shrink fitting of metal parts.
Liquid nitrogen also has a number of classified applications in the missile and space programs of the United States, in which it is used in large quantities.
| | Description | Nitrogen makes up the major portion of the atmosphere
(78.08 percent by volume, 75.5 percent
by weight). It is a colorless, odorless,
tasteless, nontoxic, almost totally inert gas, and
is colorless as a liquid. Nitrogen is nonflammable,
will not support combustion, and is not life
supporting. It combines with some of the more
active metals such as lithium and magnesium to
form nitrides, and at high temperatures it will
also combine with hydrogen, oxygen, and other
elements. It is used as an inert protection against
atmospheric contamination in many nonwelding
applications. Nitrogen is only slightly soluble in
water and most other liquids, and is a poor conductor
of heat and electricity. As a liquid at
cryogenic temperatures it is nonmagnetic. It is
shipped as a nonliquefied gas at pressures of
2000 psig (13 790 kPa) or above, and also as a
cryogenic fluid at pressures and temperatures
below 200 psig (1380 kPa) and -261°F
(-163°C). | | Chemical Properties | Solid nitrogen has a hexagonal crystal structure. Nitrogen at standard conditions is a colorless, odorless, tasteless gas. The gas is slightly soluble in H2O (2.35 parts nitrogen in 100 parts H2O at 0°C), the solubility decreasing with increasing temperature (1.55 parts nitrogen in 100 parts H2O at 20°C). Nitrogen is slightly soluble in alcohol and is essentially insoluble in most other known liquids. | | Chemical Properties | Nitrogen occurs naturally as approximately 78% v/v of the atmosphere. It is a nonreactive, noncombustible, colorless, tasteless, and odorless gas. It is often used under refrigeration as a cryogenic liquid. The boiling point is -195.8 °C and -320 °F. Nitrogen is not combustible. Nitrogen can combine with oxygen at high temperatures to form oxides and may form ammonia in contact with hydrogen at elevated temperatures. Cyanides can form if nitrogen is heated with carbon in presence of alkalies or barium oxide. If nitrogen comes in contact with ozone, nitrogen can oxidize explosively.It is usually handled as a compressed gas, stored in metal cylinders.
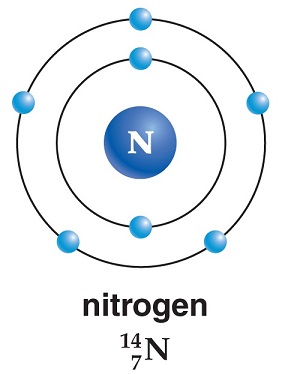 | | Physical properties | In its natural gaseous state, nitrogen is a relatively inert diatomic molecule (N2) that iscolorless, odorless, and tasteless, yet it is responsible for hundreds of active compounds. Itmakes up about 78% of the air we breathe. We are constantly taking it into our lungs withno stimulation or sensation; therefore, we really do not detect its presence. When liquefied, itis still colorless and odorless and resembles water in density. The melting point of nitrogen is–209.86°C, its boiling point is –195.8°C, and its density as a gas is 0.0012506 g/cm3. | | Isotopes | There are 19 isotopes of nitrogen, two of which are stable. The stable ones andtheir proportion to the natural abundance of nitrogen on Earth follow: N-14 = 99.634%and N-15 = 0.366%. The other 17 isotopes are radioactive and man-made in nuclearreactors and have half-lives ranging from a few nanoseconds to 9.965 minutes. | | Origin of Name | From the two Greek words nitron and genes, which together stand for
“soda or saltpeter forming.” | | Occurrence | Nitrogen is the 30th most abundant element on Earth. There is an almost unlimited sourceof nitrogen available to us considering that our atmosphere constitutes 4/5, or over 78%, ofthe nitrogen by volume. Over 33 million tons of nitrogen is produced each year by liquefyingair and then using fractional distillation to produce nitrogen as well as other gases in the atmosphere. During this process the air is cooled and then slowly warmed to fractionaltemperature points at which each specific gas in the air will “boil” off. (Note: Oxygen, argon,carbon dioxide, and nitrogen all have specific boiling points and these gases can be used tocollect the specific gas during the fractionation process.) When the temperature –reaches–195.8°C, the nitrogen is boiled off and collected.
There is a balance of nitrogen with other gases in the atmosphere that is maintained bywhat is called the nitrogen cycle. This cycle includes several processes, including nitrogen fixationof bacteria in the soil by legumes (bean and pea plants). Lightning produces nitrogen, asdo industrial waste gases and the decomposition products of organic material (i.e., organicproteins and amino acids in plants and animals contain nitrogen). In time, these sourcesreplace the nitrogen in the atmosphere to complete the cycle.
Ammonia (NH3) is the first binary molecule discovered in outer space of our galaxy, theMilky Way. It may also be the main compound that forms the rings of the planet Saturn. | | Characteristics | There are approximately 4,000 trillion tons of gas in the atmosphere, and nitrogen makesup about 78% of these gases. It is slightly soluble in water and alcohol. It is noncombustibleand is considered an asphyxiant gas (i.e., breathing pure nitrogen will deprive the body ofoxygen).
Although nitrogen is considered an inert element, it forms some compounds that are veryactive. Of the diatomic molecules, such as CO2 , it is difficult to separate the two atoms innitrogen’s molecules because of their strong binding energy. This is the reason that, along withcarbon dioxide, nitrogen gas is stable. However, once separated, the individual atoms of nitrogen(N) become very reactive and do combine with hundreds of other elements.
Nitrogen can be liquefied easily, making it useful in many applications wherein sustainedcooling is needed. At high temperatures, nitrogen reacts with many metals to form nitrides. | | Uses | Nitrogen has many uses. It is the second most commonly produced chemical in the UnitedStates. Its chemical and physical properties, along with the five electrons in its outer shell,make it a versatile element that can react as a metal or nonmetal to produce numerous compounds.Some of its uses are based on its inertness as a gas (N2) and its ability to be liquefiedto provide very low temperatures.
When recovered as a gas in the atmosphere, it is used to produce anhydrous ammonia(NH3), which is the fifth most commonly produced chemical in the United States. It is alsoused as the basis for making many nitrogen compounds. At one time it was believed to beimpossible to combine hydrogen with nitrogen to form ammonia, a natural product of animalwaste that was used as a fertilizer and textile bleach, among other things.
Nitric acid (HNO3) is an important commercial chemical and was manufactured commerciallyto produce fertilizers and explosives as well as plastics and many other products. In1902 a German chemist, Wilhelm Ostwald (1853–1932), developed a process wherein at hightemperatures he used platinum catalysts to convert ammonia into nitric acid. When nitric acidis reacted with glycerol, the result is nitroglycerine—an unstable explosive unless dissolved ininert material, such as clay. It can then be stabilized as dynamite. | | Uses | Nitrogen serves the important function of diluent in the earth’s atmosphere, controlling natural burning and respiration rates that otherwise would proceed much faster with higher concentrations of oxygen. Nitrogen is an important ingredient of numerous inorganic and organic compounds, including alkaloids, amides, amines, cyanides, cyanogens, diazo compounds, hydrazines, imides, nitrates, nitrides, nitrites, nitriles, oximes, purines, pyridines, and ureas. In terms of high-tonnage production, the nitrogen compound NH3 (ammonia) ranks first with worldwide production exceeding 50 million tons annually. | | Uses | In manufacture of ammonia, nitric acid, nitrates, cyanides, etc.; in manufacture of explosives; in filling high-temp thermometers, incandescent bulbs; to form an inert atm for preservation of materials, for use in dry boxes or glove bags. Liquid nitrogen in food-freezing processes; in the laboratory as a coolant. Pharmaceutic aid (air displacement). | | Definition | Nitrogen, N2, is a colorless,odorless, inert gas that comprises 80%of the earth's atmosphere. It serves as a diluent and controls natural burning and respiration rates, which would be much faster in higher concentrations of oxygen. Nitrogen is soluble in water and alcohol, but is essentially insoluble in most other liquids. It is essential to practically all forms of life and its compounds serve as foods or fertilizers. Nitrogen is used in the manufacture of ammonia and nitric acid. Nitrogen is essentially an inert gas at ambient and moderate temperatures. Therefore, it is easily handled by most metals.At elevated temperatures, nitrogen can be aggressive to metals and alloys. | | Definition | Dinitrogen: the normal form ofmolecular nitrogen, N2 used to distinguishit from nitrogen atoms. | | Definition | nitrogen: Symbol N. A colourlessgaseous element belonging to group15 (formerly VB) of the periodictable; a.n. 7; r.a.m. 14.0067; d. 1.2506g dm–3; m.p. –209.86°C; b.p. –195.8°C.It occurs in air (about 78% by volume)and is an essential constituent of proteinsand nucleic acids in living organisms.Nitrogen is obtained for industrialpurposes by fractional distillation ofliquid air. Pure nitrogen can be obtainedin the laboratory by heating a metal azide. There are two naturalisotopes: nitrogen–14 and nitrogen–15 (about 3%). The element isused in the Haber process for makingammonia and is also used to providean inert atmosphere in weldingand metallurgy. The gas is diatomicand relatively inert – it reacts withhydrogen at high temperatures andwith oxygen in electric discharges. Italso forms nitrides with certainmetals. Nitrogen was discovered in1772 by Daniel Rutherford (1749–1819). | | Production Methods | Nitrogen is obtained commercially, in large quantities, by the
fractional distillation of liquefied air. | | General Description | A colorless odorless gas. Noncombustible and nontoxic. Makes up the major portion of the atmosphere, but will not support life by itself. Used in food processing, in purging air conditioning and refrigeration systems, and in pressurizing aircraft tires. May cause asphyxiation by displacement of air. Under prolonged exposure to fire or heat containers may rupture violently and rocket. | | Air & Water Reactions | Slightly soluble in water. | | Reactivity Profile | These substances undergo no chemical reactions under any known circumstances except those under extreme conditions (liquid Nitrogen reacts violently in mixture with magnesium powder when a fuse is lit. Due to formation of magnesium nitride). Otherwise, they are nonflammable, noncombustible and nontoxic. They can asphyxiate. | | Hazard | Nitrogen is nontoxic, but it is an asphyxiate gas that cannot, by itself, support oxidation(combustion) or support life. If you breathe pure nitrogen for any period of time, you will die—not because the nitrogen gas is a poison, but because your body will be deprived of oxygen.
Nitrogen oxides are formed under certain conditions when nitrogen combines with oxygen,thus contributing to pollution. One source is from the internal combustion engine thatproduces NO similar to lightning. Once released, it combines with more oxygen to form ,which is a very reactive polluting gas. Nitrogen dioxide NO2 is the main cause of “brown”smog over some cities and is harmful to plants, animals, and humans. To make matter worse,if there is adequate sunlight at the time of the smog, the ultraviolet light of the sun will breakdown the N and O of the NO2 to form free radicals of oxygen that are reactive, forming ozone(O3), which is itself a strong oxidizing agent that adds to pollution.
Several of the oxygen, hydrogen, and halogen compounds of nitrogen are toxic wheninhaled. A common error made in using household cleaners is to mix or use together ammoniacleaning fluids (containing nitrogen) and Clorox-type cleaning fluids (containing chlorine).The combined fumes can be deadly in any confined area. NEVER mix Clorox with ammoniatypecleaning fluids. | | Health Hazard | Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. | | Fire Hazard | Non-flammable gases. Containers may explode when heated. Ruptured cylinders may rocket. | | Agricultural Uses | plant growth. It is a gaseous element of Group 15
(formerly VB) of the Periodic Table, having an atomic
number of 7 There are two stable and four
radioactive isotopes of nitrogen. Nitrogen is a part of all
amino acids, proteins, chlorophylls, enzymes, alkaloids,
phosphotides, vitamins, hormones, nucleic acid and
other plant substances. With as much as 78% in the
atmosphere and 98% in the soil organic matter, nitrogen
is abundant in nature. Yet, it is most deficient in all
cultivated soils because (a) nitrogen is lost in many ways,
(b) both microbes and plants compete for soil nitrogen,and (c) soil has little capacity to hold nitrogen in oxidized
forms. With all vital processes being associated with
functionally reactive plasma in the nitrogen-containing
proteins, it is obvious that life is inconceivable without
nitrogen.
Nitrogen in adequate quantity often leads to the
desirable thin cell walls and leads to more tender and
succulent plants, resulting into a better crop yield.
Nitrogen is absorbed by plants either in the cationic or the
anionic form as ammonium ion (NH4+ ) or nitrate ion
(NO3 - ). These ions are soluble in water and are,
therefore, very easily leached. If fertilizer is applied
when it rains, obviously a lot of it will be washed away,
and in this way, the annual nitrogen loss can be as much
as 50 to 80 kg/ha.
Nitrogen loss occurs through leaching, volatilization,
immobilization and ammonium faation. Denitrification
or conversion of nitrate to nitrogen gas by bacteria is
another cause for extensive loss of gaseous nitrogen.
Ammonium ions in a basic solution leads to ammonia loss
by volatilization. Surface applications of any ammonium
or urea fertilizer on calcareous soils cause the largest
ammonia losses.
The mineralized ammonium ions have a very short
life, whereas the nitrification process is rapid. So,
slowing down of the oxidation of ammonium ions to the
nitrate form reduces the nitrate (and nitrogen) loss by
leaching or denitrification. Several nitrification
inhibitors such as nitrapyrin and dicyandiamide (DCD)
are used to inhibit nitrification.
Nitrogen furaton provides a major source of soil
nitrogen. Nitrogen fixation involves the action of
microbes that convert the relatively inert nitrogen of the
soil air into such forms as are useful to plants. The natural
biological and chemical processes through which
inorganic and organic nitrogen are inter-converted, are
collectively known as the nitrogen cycle. It includes
ammonification, ammonia assimilation, nitrification,
nitrate assimilation, nitrogen fixation and denitrification.
Materials supplying nitrogen are (a) anhydrous
ammonia (NH3) which is hazardous and difficult to
handle, (b) urea [CO(NH2)2] which is a good, cheap and
the most popular fertilizer, (c) ammonium nitrate
(NH4NO3) which is a relatively cheap source of solid
nitrogen fertilizer, and (d) ammonium sulphate
[(NH4)2SO4] which is not as popular as urea and
ammonium nitrate.
Since fertilizer nitrogen efficiency is determined by
the biomass yield and nitrogen uptake by the crop, all
factors affecting these also affect the efficiency of
nitrogen usage. These factors are classified into five
groups such as the soil, crop, environment, agronomic
practices and fertilizer management.
Nitrogen deficiency symptoms are most prevalent
and the easiest to identify. Young plants exhibit yellowish
green foliage and stunted growth while older plants show
yellowing or falling of leaves.
Nitrogen deficiency impedes good yield. An
effective, integrated approach employs organic manures,
biofertilizers, chemical fertilizers, nitrification
inhibitors, coated and long-persisting nitrogen
fertilizers. Such practices hold the key to sustainable
agriculture. Nitrogen is used in the production of
ammonia, acrylonitrile, nitrates, cyanamide, cyanides
and nitrides. It is used in the manufacture of explosives
and as an inert gas for purging. It is also used in cryogenic
preservation, as a source of pressure in oil wells,
inflating tires and as a component of fertilizer mixtures.
However, overuse of nitrogen fertilizers is responsible
for increased quantities of nitrates in the soil water,
posing a serious threat to the environment. | | Pharmaceutical Applications | Nitrogen and other compressed gases such as carbon dioxide and
nitrous oxide are used as propellants for topical pharmaceutical
aerosols. They are also used in other aerosol products that work
satisfactorily with the coarse aerosol spray produced with
compressed gases, e.g. furniture polish and window cleaner.
Nitrogen is insoluble in water and other solvents, and therefore
remains separated from the actual pharmaceutical formulation.
Advantages of compressed gases as aerosol propellants are that
they are less expensive; of low toxicity; and practically odorless and
tasteless. In contrast to liquefied gases, their pressures change
relatively little with temperature. However, there is no reservoir of
propellant in the aerosol and as a result the pressure decreases as the
product is used, changing the spray characteristics.
Misuse of a product by the consumer, such as using a product
inverted, results in the discharge of the vapor phase instead of the
liquid phase. Most of the propellant is contained in the vapor phase
and therefore some of the propellant will be lost and the spray characteristics will be altered. Additionally, the sprays produced
using compressed gases are very wet. However, recent developments
in valve technology have reduced the risk of misuse by making
available valves which will spray only the product (not propellant)
regardless of the position of the container. Additionally, barrier
systems will also prevent loss of propellant, and have been used for
pharmaceuticals and cosmetic aerosol sprays and foams utilizing
nitrogen as the propellant.
Nitrogen is also used to displace air from solutions subject to
oxidation, by sparging, and to replace air in the headspace above
products in their final packaging, e.g. in parenteral products
packaged in glass ampoules. Nitrogen is also used for the same
purpose in many food products. | | Industrial uses | Nitrogen is often called an inert gas, and is used for some inert atmospheres for metal treating and in lightbulbs to prevent arcing, but it is not chemically inert. It is a necessary element in animal and plant life, and is a constituent of many useful compounds. Nitrogen combines with many metals to form hard nitrides useful as wear-resistant metals. Small amounts of nitrogen in steels inhibit grain growth at high temperatures, and also increase the strength of some steels. It is also used to produce a hard surface on steels.
Because of the importance of nitrogen compounds in agriculture and chemical industry, much of the industrial interest in elementary nitrogen has been in processes for converting elemental nitrogen into nitrogen compounds. The principal methods for doing this are the direct synthesis of ammonia from nitrogen and hydrogen, the electric arc process, which involves the direct combination ofN2 and O2 to nitric oxide, and the cyanamide process. | | Materials Uses | Gaseous nitrogen is noncorrosive and inert, and
may consequently be contained in systems constructed
of any common metals and designed to
safely withstand the pressures involved. At the
temperature of liquid nitrogen, ordinary carbon
steels and most alloy steels lose their ductility
and are considered unsafe for liquid nitrogen
service. Satisfactory materials for use with liquid
nitrogen include austenitic stainless steel
(for example, types 304 and 316) and other
nickel-chromium alloys, copper, Monel, brass,
and aluminum. | | Pharmacology | Atropine
does not reactivate the phosphorylated AChE but competes
with acetylcholine for binding with the muscarinic
acetylcholine receptor acting as an antagonist. | | Safety Profile | Low toxicity. In high
concentrations it is a simple as-p~h yxiant.
The release of nitrogen from solution in the
blood, with formation of small bubbles, is
the cause of most of the symptoms and
changes found in compressed air illness
(caisson disease). It is a narcotic at hgh
concentration and hgh pressure. Both the
narcotic effects and the bends are hazards of
compressed air atmospheres such as found
in underwater dving. Nonflammable gas.
Can react violently with lithium,
neodymium, titanium under the proper
condtions. See also ARGON. | | Safety | Nitrogen is generally regarded as a nontoxic and nonirritant
material. However, it is an asphyxiant and inhalation of large
quantities is therefore hazardous. | | Potential Exposure | Nitrogen is present in the air we
breathe. Health effects may occur at concentrations above 80%. It has many medical and industrial uses including the
quick freezing of food. The gas is used for purging, heat
treating; food freezing; annealing, cooling, oil recovery; in
the inert blanketing of sensitive materials and as a reactant
in chemical synthesis of ammonia. | | Physiological effects | Nitrogen is nontoxic and largely inert. It can act
as a simple asphyxiant by diluting the concentration
of oxygen in air below levels necessary
to support life. Inhalation of nitrogen in excessive
concentrations can result in dizziness, nausea,
vomiting, loss of consciousness, and death.
Death may result from errors in judgment,
confusion, or loss of consciousness, which prevents
self-rescue. At low-oxygen concentrations,
unconsciousness and death may occur in
seconds without warning.
Gaseous nitrogen must be handled with all the
precautions necessary for safety with any nonflammable,
nontoxic compressed gas.
All precautions necessary for the safe handling
of any gas liquefied at very low temperatures
must be observed with liquid nitrogen.
Extensive tissue damage or burns can result from exposure to liquid nitrogen or cold nitrogen
vapors. | | storage | Nitrogen is stable and chemically unreactive. It should be stored in
tightly sealed metal cylinders in a cool, dry place. | | Shipping | UN1066 Nitrogen, compressed, Hazard Class:,
Hazard Class: 2.2; Labels: 2.2-Nonflammable compressed
gas; UN1977 Nitrogen, refrigerated liquid cryogenic liquid,
Hazard Class:, Hazard Class: 2.2; Labels: 2.2-
Nonflammable compressed gas. Cylinders must be transported
in a secure upright position, in a well-ventilated
truck. Protect cylinder and labels from physical damage.
The owner of the compressed gas cylinder is the only entity
allowed by federal law (49CFR) to transport and refill
them. It is a violation of transportation regulations to refill
compressed gas cylinders without the express written permission
of the owner. | | Purification Methods | Cylinder N2 can be freed from oxygen by passage through Fieser's solution [which comprises 2g sodium anthraquinone-2-sulfonate and 15g sodium hydrosulfite dissolved in 100mL of 20% KOH; see Fieser, J Am Chem Soc 46 2639 1924] followed by scrubbing with saturated lead acetate solution (to remove any H2S generated by the Fieser solution), conc H2SO4 (to remove moisture), then soda-lime (to remove any H2SO4 and CO2). Alternatively, after passage through Fieser's solution, N2 can be dried by washing with a solution of the metal ketyl from benzophenone and Na wire in absolute diethyl ether. [If ether vapour in N2 is undesirable, the ketyl from liquid Na-K alloy under xylene can be used.] Another method for removing O2 is to pass the nitrogen through a long, tightly packed column of Cu turnings, the surface of which is constantly renewed by scrubbing it with ammonia (sg 0.880) solution. The gas is then passed through a column packed with glass beads moistened with conc H2SO4 (to remove ammonia), through a column of packed KOH pellets (to remove H2SO4 and to dry the N2), and finally through a glass trap packed with chemically clean glass wool immersed in liquid N2. Nitrogen has also been purified by passage over Cu wool at 723oK and Cu(II) oxide [prepared by heating Cu(NO3)2.6H2O at 903oK for 24hours] and then into a cold trap at 77oK. A typical dry purification method consists of a mercury bubbler (as trap), followed by a small column of silver and gold turnings to remove any mercury vapour, towers containing anhydrous CaSO4, dry molecular sieves or Mg(ClO4)2, a tube filled with fine Cu turnings and heated to 400o by an electric furnace, a tower containing soda-lime, and finally a plug of glass wool as filter. Variations include tubes of silica gel, traps containing activated charcoal cooled in a Dry-ice bath, copper on Kieselguhr heated to 250o, and Cu and Fe filings at 400o. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 458-460 1963.] | | Incompatibilities | Containers may explode when heated.
Liquid nitrogen is very unreactive, nonflammable, noncombustible
and nontoxic. Contact with water may result in
vigorous or violent boiling and extremely rapid vaporization.
If the water is hot, there is the possibility that a liquid
“superheat” explosion may occur. Pressures may build to
dangerous levels if the liquid contacts water in a closed
container. | | Waste Disposal | Return refillable compressed
gas cylinders to supplier. Vent to atmosphere. | | Regulatory Status | GRAS listed. Included in the FDA Inactive Ingredients Database
(injections; dental preparations; nasal sprays; oral solutions; rectal
gels). Accepted for use as a food additive in Europe. Included in
parenteral and nonparenteral medicines licensed in the UK and
USA. Included in the Canadian List of Acceptable Non-medicinal
Ingredients. |
| | Nitrogen Preparation Products And Raw materials |
|