10035-16-2
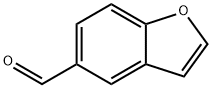 10035-16-2 结构式
10035-16-2 结构式
基本信息
5-FORMYLBENZO[BETA]FURAN
5-FORMYLBENZO(B)FURAN
5-Formylbenzofuran
Benzofuran-5-carboxaldehyde
Benzofurancarbaldehyde
Benzo[b]furan-5-carboxaldehyde 96%
5-Formylbenzo[beta]furan, min. 96 %
Benzo[b]furan-5-carboxaldehyde
5-Benzofurancarbaldehyde
Benzofuran-5-carbaldehyde
物理化学性质
制备方法
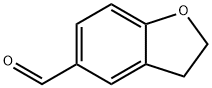
55745-70-5

10035-16-2
以2,3-二氢苯并呋喃-5-甲醛为原料合成苯并呋喃-5-甲醛的一般步骤:在室温和氩气保护下,将N-溴代琥珀酰亚胺(NBS,0.656 g,3.69 mmol)和偶氮二异丁腈(AIBN,8.10 mg,49.2 μmol)加入到2,3-二氢苯并呋喃-5-甲醛(0.364 g,2.46 mmol)的氯苯(7.3 mL)溶液中。反应混合物在80℃下搅拌1小时后,冷却至室温,并用乙酸乙酯(EtOAc)稀释。有机层依次用饱和碳酸氢钠(NaHCO3)水溶液和盐水洗涤,经无水硫酸钠(Na2SO4)干燥后过滤,滤液在减压下浓缩。残余物通过硅胶柱色谱(洗脱剂:乙酸乙酯/己烷,3:97)纯化,得到苯并呋喃-5-甲醛(0.268 g,1.84 mmol,产率75%),为无色油状物。其1H-NMR(CDCl3,400 MHz)数据如下:δ 6.82(d,J = 2.0 Hz,1H,呋喃-H),7.54(d,J = 8.8 Hz,1H,Ar-H),7.65(d,J = 2.0 Hz,1H,呋喃-H),7.79(d,J = 8.8 Hz,1H,Ar-H),8.07(s,1H,Ar-H),9.99(s,1H,-CHO)。所得化合物的光谱数据与文献报道(van Otterlo et al., 2005)一致。
参考文献:
[1] Journal of Medicinal Chemistry, 2009, vol. 52, # 20, p. 6270 - 6286
[2] Phytochemistry, 2013, vol. 96, p. 132 - 147
[3] Patent: US2014/309427, 2014, A1. Location in patent: Paragraph 0074; 0075; 0076; 0077
[4] Patent: WO2018/64135, 2018, A1. Location in patent: Paragraph 0174-0177

