210644-62-5
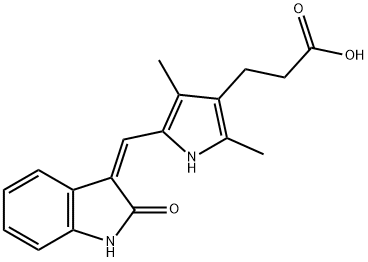 210644-62-5 结构式
210644-62-5 结构式
基本信息
(Z)-3-(2,4-二甲基-5-((2-氧代吲哚啉-3-亚基)甲基)-1H-吡咯-3-基)丙酸
5-[(Z)-(1,2-二氢-2-氧代-3H-吲哚-3-亚基)甲基]-2,4-二甲基-1H-吡咯-3-丙酸
(Z)-SU6668
(Z)-TSU-68
(Z)-Orantinib
PDGFR Tyrosine Kinase Inhibitor VI, SU6668
PDGFR Tyrosine Kinase Inhibitor VI, SU6668 - CAS 210644-62-5 - Calbiochem
3-[2,4-dimethyl-5-[(Z)-(2-oxo-1H-indol-3-ylidene)methyl]-1H-pyrrol-3-yl]propanoicacid
3-(2,4-Dimethyl-5-{[(3Z)-2-oxo-1H-indol-3-ylidene]methyl}-1H-pyrrol-3-yl)propanoic acid
3-(2,4-Dimethyl-5-(2-oxo-1,2-dihydroindol-3-ylidenemethyl)-1H-pyrrol-3-yl)-propionic acid
5-((Z)-1,2-Dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-propanoic acid
物理化学性质
制备方法
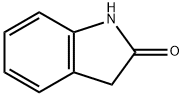
59-48-3
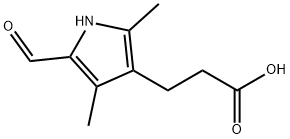
1133-96-6

210644-62-5
以3-(5-甲酰基-2,4-二甲基-1H-吡咯-3-基)丙酸(10g,51mmol)、2-吲哚酮(6.5g,49mmol)和氢氧化钠(40g,58mmol)为原料,将混合物溶解于50ml水中,在50℃下搅拌反应4小时。反应完成后,将混合物冷却至室温,过滤,滤液用12N盐酸酸化至pH3。通过真空过滤收集沉淀的固体,用10ml水洗涤,并在真空下干燥过夜。粗产物用热乙醇洗涤两次,然后通过真空过滤收集固体,用10ml乙醇洗涤,并在真空下干燥,得到13.8g(产率91%)的(Z)-3-(2,4-二甲基-5-((2-氧代吲哚啉-3-亚基)甲基)-1H-吡咯-3-基)丙酸。1HNMR(360MHz,DMSO-d6)数据如下:δ13.38(s,br,1H,NH-1'),12.05(s,br,1H,COOH),10.70(s,br,1H,NH-1),7.69(d,J=7.39Hz,1H,H-4),7.53(s,1H,H-乙烯基),7.06(t,J=7.39Hz,1H,H-6),6.95(t,J=7.39Hz,1H,H-5),6.85(d,J=7.39Hz,1H,H-7),2.63(t,J=7.45Hz,2H,CH2CH2COOH),2.34(t,J=7.45Hz,2H,CH2CH2COOH),2.28(s,3H,CH3),2.24(s,3H,CH3)。质谱数据:MS m/z 311([M+1]+,100)。
参考文献:
[1] Patent: US6878733, 2005, B1. Location in patent: Page/Page column 215
[2] Patent: US6878733, 2005, B1. Location in patent: Page/Page column 215
[3] Journal of Heterocyclic Chemistry, 2003, vol. 40, # 1, p. 181 - 185
[4] Journal of Medicinal Chemistry, 1999, vol. 42, # 25, p. 5120 - 5130
[5] Patent: US6395734, 2002, B1