28783-41-7
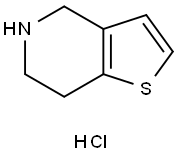 28783-41-7 结构式
28783-41-7 结构式
基本信息
4,5,6,7-四氢噻吩并[3,2-c]吡啶 盐酸盐
4,5,6,7-四氢噻吩[3,2,c]吡啶盐酸盐
4,5,6,7-四氢噻吩[3,2-C]吡啶
4,5,6,7-四氢二噻吩基[3,2-C]盐酸吡啶,96%
4,5,6,7-TETRAHYDROTHIENO[3,2-C]PYRIDINE HYDROCHLORIDE
4,5,6,7-tetrahydrotieno[3,2-c]pyridinium chloride
4,5,6,7-TETRAHYDROTHIENO[3,2-C]PYRIDINE HCl(TTP)
4,5,6,7-tetrahydrothieno[3,2-c]pyridinium chloride
Tetrahydrothieno pyridine HCl
4,5,6,7-Tetrahydrothieon[3,2-C]pyridine,HCl
4,5,6,7-Tetrahydrothieon[3,2-C]pyridine,hydrochloride
6,7-Dihydro-4H-thieno[3,2-c]pyridine Hydrochloride
4,5,6,7-TET RAH YDROTHIENO[3,2-C]PYRIDE
4,5,6,7-Tetrahydrothiophene[3,2-c]pyridine hydrochloride
4,5,6,7-Tetrahydrothieno[3,2-c]pyridine hydrochloride ,98%
物理化学性质
安全数据
toxic compounds or compounds which causing chronic effects
制备方法
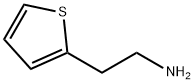
30433-91-1

50-00-0
![4,5,6,7-四氢噻吩[3,2-c]吡啶盐酸盐](/CAS/GIF/28783-41-7.gif)
28783-41-7
实施例5:制备4,5,6,7-四氢噻吩[3,2-c]吡啶盐酸盐 在配备温度计和机械搅拌器的2升四颈烧瓶中,加入2-噻吩乙胺(100g,0.79摩尔)。于25℃(±5℃)下,将反应物溶解于二氯甲烷(600ml)中,搅拌5至10分钟。随后,向反应体系中加入多聚甲醛(26.4g,0.88摩尔),并在40至45℃下进行共沸回流反应4至6小时。反应完成后,将体系冷却至室温,并于25℃(±5℃)下,缓慢加入7%盐酸的N,N-二甲基甲酰胺溶液(200ml)。将反应混合物在70℃(±5℃)下继续搅拌4至6小时。之后,将反应液冷却至15℃(±2℃),并搅拌8至10小时以促进结晶。过滤收集固体产物,并用预冷的二氯甲烷洗涤。最后,将产物在30至40℃下真空干燥,得到4,5,6,7-四氢噻吩[3,2-c]吡啶盐酸盐(120g,收率99%)。
参考文献:
[1] Patent: WO2012/1486, 2012, A1. Location in patent: Page/Page column 18
[2] Journal of Organic Chemistry, 2015, vol. 80, # 14, p. 7019 - 7032
[3] Patent: EP1772455, 2007, A2. Location in patent: Page/Page column 9
[4] Journal of Organometallic Chemistry, 2009, vol. 694, # 13, p. 2092 - 2095
[5] Patent: US2004/24011, 2004, A1. Location in patent: Page 7
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | T2770 | 4,5,6,7-四氢噻吩并[3,2-c]吡啶盐酸盐 4,5,6,7-Tetrahydrothieno[3,2-c]pyridine Hydrochloride | 28783-41-7 | 5g | 120元 |
| 2025/12/22 | T2770 | 4,5,6,7-四氢噻吩并[3,2-c]吡啶盐酸盐 4,5,6,7-Tetrahydrothieno[3,2-c]pyridine Hydrochloride | 28783-41-7 | 25g | 390元 |
| 2025/05/22 | XW287834175 | 4,5,6,7-四氢噻吩并[3,2-C]吡啶盐酸盐 4,5,6,7-tetrahydrothieno[3,2,c]pyridine hydrochloride;4,5,6,7-tetrahydrothieno[3,2-c]pyridinium chloride | 28783-41-7 | 5G | 31元 |
