Palladium(II) acetat Chemische Eigenschaften,Einsatz,Produktion Methoden
R-Sätze Betriebsanweisung:
R41:Gefahr ernster Augenschäden.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R40:Verdacht auf krebserzeugende Wirkung.
S-Sätze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S39:Schutzbrille/Gesichtsschutz tragen.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Chemische Eigenschaften
Brown needles, soluble in benzene and toluene, insoluble in ether and alcohol, keep in dark and refrigerated.
Verwenden
Palladium (II) Acetate Trimer is used in Suzuki-Miyaura cross-coupling reactions and a catalyst for intramolecular coupling. It also serves to catalyze the chemoselective reduction of nitroarenes.
Definition
Palladium acetate is a chemical compound of palladium. It is used as a catalyst for many organic reactions and as a precursor to other palladium(II) compounds. Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is found as a free metal alloyed with gold and other platinum group metals and in the rare minerals cooperite and polarite. (Toxin and Toxin Target Database (T3DB))
synthetische
The synthesis of palladium acetate was first reported by Wilkinson et al.,where glacial acetic acid containing palladium powder is heated at reflux with a minimal amount of nitric acid until the generation of brown NOx fumes ceases. Although this is a relatively old method, it is still the most commercially employed route for the synthesis of palladium acetate, considering the process economics. Palladium (II) Acetate can be synthesized in two steps from palladium sponge via the intermediate dinitrate by first combining it with hot glacial acetic acid and nitric acid:
Pd + 2 HNO3 → Pd(NO3)2 + H2
Pd(NO3)2 + 2 CH3COOH → Pd(O2CCH3)2 + 2 HNO3
Reaktionen
-
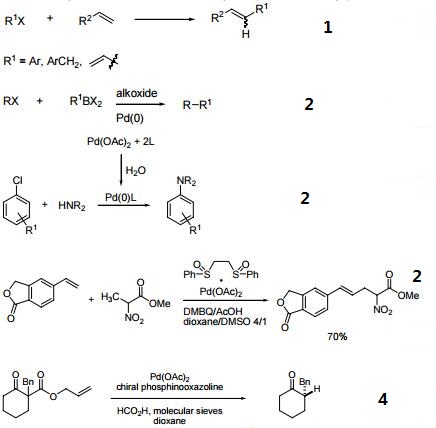
Efficient catalyst for the arylation of olefins (Heck reaction).
-
Catalyst for cross-coupling reactions.
-
Catalyst for C-H activation.
-
Precatalyst for enantioselective decarboxylative protonation of allyl β-ketoesters.
Allgemeine Beschreibung
Palladium(II) acetate is a homogenous oxidation catalyst. It participates in the activation of alkenic and aromatic compounds towards oxidative inter- and intramolecular nucleophilic reactions. Crystals of palladium(II) acetate have a trimeric structure, having symmetry
D3h. Each of the palladium atoms in the crystals are joined to the other two by double acetate bridges. Microencapsulation of palladium(II) acetate in polyurea affords polyurea-encapsulated palladium(II) acetate. It is a versatile heterogeneous catalyst for various phosphine-free cross-coupling reactions. It participates as catalyst in the Heck coupling reaction of pthalides with different alkenes.
Sicherheitsprofil
Moderately toxic by ingestion. When heated to decomposition it emits toxic vapors of palladium.
läuterung methode
It recrystallises from CHCl3 as purple crystals. It can be washed with AcOH and H2O and dried in air. Large crystals are obtained by dissolving it in *C6H6, adding half its volume of AcOH and allowing it to evaporate slowly at room temperature. It forms green adducts with nitrogen donors, it dissolves in KI solution to form solid PdI2 and a red solution of PdI42-, but is insoluble in aqueous saturated NaCl, and NaOAc. It dissolves in HCl to form PdCl42-. It is soluble in CHCl3, CH2Cl2, Me2CO, MeCN, Et2O, but it is insoluble in H2O, and decomposes when warmed in alcohols in which it is also insoluble. [Morehouse et al. Chem Ind (London) 544 1964, Stephenson et al. J Chem Soc 3632 1965, Skapski & Smart J Chem Soc (D) 658 1970, Heck Acc Chem Res 12 146 1979.]
Palladium(II) acetat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
3-AMINOBIPHENYL
2,3-DIHYDRO-1H-INDOLE-5-CARBOXYLIC ACID
3-PHENYLBENZYLAMINE
N,N'-Di-1-naphthyl-N,N'-diphenylbenzidine (NPB)
5-CYANO-2,3-DIHYDRO-1H-INDOLE
Ethyl (R)-2-hydroxy-4-phenylbutyrate
(E)-METHYL 3-(4-BROMOPHENYL)ACRYLATE
(S)-(-)-7,7'-BIS[DI(3,5-DIMETHYLPHENYL)PHOSPHINO]-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
5-PHENYL-2-THIOPHENECARBALDEHYDE
C-(3,4-DIHYDRO-2H-BENZO[1,4]OXAZIN-3-YL)-METHYLAMINE
(S)-7,7'-Bis[di(p-methylphenyl)phosphino]-1,1'-spirobiindane ,97%
4-(2'-Oxobenzimidazolin-1'-yl)piperidin
1-ACETYL-5-AMINO-2,3-DIHYDRO-(1H)-INDOLE
ethyl 1H-indole-6-carboxylate
(R)-(+)-7,7'-BIS[DI(4-METHYLPHENYL)PHOSPHINO]-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
2-MORPHOLINO-5-(TRIFLUOROMETHYL)BENZALDEHYDE
4-Aminophenylboronic acid pinacol ester
(R)-(+)-7,7'-BIS(DIPHENYLPHOSPHINO)-2,2',3,3'-TETRAHYDRO-1,1'-SPIROBIINDANE
Diphenylacetylen
ethyl indoline-6-carboxylate
5-Phenylthiophene-2-carboxylic acid
4-Methoxy-7H-furo[3,2-g][1]benzopyran-7-on
4-Aminobiphenyl
INDOLINE-6-CARBOXYLIC ACID
3-Phenyl-1H-pyrazole
1-(4-Pyridyl)piperazin
Allylacetat
5-Methylindol
4,6-Diamino-2-pyrimidinol
(R)-7,7'-BIS(DIPHENYLPHOSPHINO)-1,1'-SPIROBIINDANE

