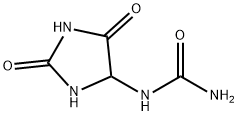
Allantoin
- CAS No.
- 97-59-6
- Chemical Name:
- Allantoin
- Synonyms
- ALL;Allatoin;GLYOXYLDIUREIDE;5-UREIDOHYDANTOIN;ALANTOIN;Egopsoryl;GLYOXYLIDIUREIDE;N-(2,5-Dioxo-4-imidazolidinyl)urea;toin;nsc7606
- CBNumber:
- CB3215461
- Molecular Formula:
- C4H6N4O3
- Molecular Weight:
- 158.12
- MDL Number:
- MFCD00005260
- MOL File:
- 97-59-6.mol
- MSDS File:
- SDS
| Melting point | 230 °C (dec.) (lit.) |
|---|---|
| Boiling point | 283.17°C (rough estimate) |
| Density | 1.6031 (rough estimate) |
| refractive index | 1.8500 (estimate) |
| Flash point | 230-234°C |
| storage temp. | 2-8°C |
| solubility | H2O: soluble0.1g/10 mL, clear, colorless |
| form | Powder |
| pka | 8.96(at 25℃) |
| color | White |
| Odor | odorless, tasteless |
| Water Solubility | Slightly soluble in water. Freely soluble in alkalis |
| Decomposition | 230-234 ºC |
| Merck | 14,258 |
| BRN | 102364 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | POJWUDADGALRAB-UHFFFAOYSA-N |
| LogP | -2.26 at 20℃ |
| FDA 21 CFR | 310.545 |
| CAS DataBase Reference | 97-59-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | ALL |
| FDA UNII | 344S277G0Z |
| NIST Chemistry Reference | Urea, (2,5-dioxo-4-imidazolidinyl)-(97-59-6) |
| EPA Substance Registry System | Allantoin (97-59-6) |
| Cosmetics Info | Allantoin |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38 | |||||||||
| Safety Statements | 22-24/25-36-26 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | YT1600000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29332100 | |||||||||
| NFPA 704 |
|
Allantoin price More Price(45)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 05670 | Allantoin ≥98.0% (N) | 97-59-6 | 25g | $51.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 05670 | Allantoin ≥98.0% (N) | 97-59-6 | 100g | $75.4 | 2024-03-01 | Buy |
| TCI Chemical | A0211 | Allantoin >98.0%(T) | 97-59-6 | 25g | $35 | 2024-03-01 | Buy |
| TCI Chemical | A0211 | Allantoin >98.0%(T) | 97-59-6 | 500g | $258 | 2024-03-01 | Buy |
| Alfa Aesar | A15571 | Allantoin, 98% | 97-59-6 | 250g | $55.2 | 2024-03-01 | Buy |
Allantoin Chemical Properties,Uses,Production
Anti-inflammatory analgesic effects
Allantoin has anti-inflammatory analgesic effects, but it also has a weak partial paralysis effect, can effectively reduce stimuli of stimulus, can be used as a skin protectant and anti-irritant, can reduce skin irritation of cosmetic ingredients, China food and Drug Administration rank it as the first kind efficient active ingredient of skin care agent, now has been widely used in many products such as shampoo, sunscreen products, creams and lotions, shaving creams and oral care products.
Chemical properties
Colorless crystalline powder. Melting point is 238-240 ℃ (decomposition). Can be dissolved in hot water, hot alcohol and dilute sodium hydroxide solution, slightly soluble in water and alcohol, almost insoluble in ether and chloroform. Odorless, tasteless. In dry air is stable, prolonged boiling in the water. or strong base will be destroyed. pH of saturated aqueous solution is 5.5.
The above information is edited by the chemicalbook of Liu Yujie.
Uses
1. Allantoin can promote skin cell growth and rapid wound healing. Used as anti-ulcer drug, mixed with dry aluminum hydroxide gel, for gastrointestinal ulcers and inflammation. The product can soften keratin, making the skin retain moisture, moist and soft, is special effects of additive in cosmetic. Allantoin and its derivatives are the quality improver and additive of many household chemical products. Allantoin protein may be formulated anti-irritant, anti-dandruff, cleaning and wound healing scalp preparations, making hair soft, shiny and elastic. The product is an amphoteric compound, can bind to a variety of material form double salts, with dark, antiseptic, analgesic, deodorant, anti-oxidation effect, and therefore is household chemical products, additives of cosmetics such as freckle cream, acne solution, shampoo , soap, toothpaste, shaving lotion, convergence liquid and antiperspirant deodorant detergent. Allantoin also is a biochemical reagents.
2. For the roles such as skin care, oral products, anti-allergy, the treatment of skin ulcers promote wound healing.
3. Widely used in treatment of a variety of skin ulcers and trauma and additives of nutritional cosmetics.
Production methods
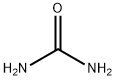
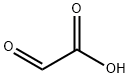
Allantoin presents in the sheath cyst fluid, fetal urine and some of the plants. However, the cost extracted Allantoin from these substances is too high. Currently synthesis methods of Allantoin are: potassium permanganate oxidation uric acid method: dichloroacetic acid and urea heated to synthesize Allantoin; direct condensation method of glyoxylic acid and urea.
1. technological process of dichloroacetic acid and urea as raw materials is as follows: The sodium methoxide solution and methanol were added into the reaction tank, and heated to 40-50 ℃, slowly dropwise added dichloroacetic acid, after added and reflux reaction for 2 h. Cooled to room temperature, filtered, washed with methanol and the filtrate was combined, washed, added methanol solution of sodium dimethoxyethane. The solution was reduced to dryness under reduced pressure , 2.8 portion of hydrochloric acid was added,heated and stirred into a paste in a water bath. Then heated to 90 ℃, and then cooled to about 10 ℃, filtered, 0.25 portion of hydrochloric acid and urea was added into , dissolved by heated, reacted at 80 ℃ for 2 h. Cooled and crystallization, and then maintained at 0 ℃ for above 3 h, centrifugation, washed ,spin-dried, dried to crude product. After The crude product was used 15 times of the water to recrystallize, Allantoin was obtained. As for the dichloroacetic acid, the total yield was 30.3%.
2. The direct condensation of glyoxylic acid and urea (glyoxylate derived by oxidation of glyoxal) operation example: (1) oxidation was in 500 ml four-hole boiling flask equipped with mechanical stirrer, condenser, thermometer and dropping funnel, added 193 g (1 mol) 30% glyoxal aqueous solution, controlled the internal temperature at 40 ± 2 ℃ and stirred to dropwise add 135.5 g ( 1 mol) 45% nitric acid, After addition, continued to stir at this temperature and reacted for 2-3 h, until reddish brown oxidation no longer escaped, the reaction solution was blue-green and immediately disappeared. Replaced by simple distillation apparatus, at about 2.76 kPa, the outside temperature did not exceed 60 ℃ and about 125 g water was evaporated, concentrate solution was standing overnight at room temperature, oxalic acid crystals were precipitated (dried to get 19 g, together 0.21 mol), concentration was 39% ). Continue to stir and react for about 8h at an internal temperature of 40 ± 2 ℃, the oxidation reaction was completed. (2) condensation In 500 ml three-hole boiling flask equipped with mechanical stirrer, condenser and thermomete, above 150 g glyoxylic acid aqueous solution was added, added 170 g (2.83 mol) urea and 23.3 g of concentrated hydrochloric acid, heated to an internal temperature of 80 ℃ under stirring. After about 15min urea was dissolved, the reaction solution was transparent, approximately another 30-45 min, the reactant appeared white cloudy, white precipitate was gradually increased, stirred at an inner temperature of 80 ℃ for 1 h, heating was stopped and the reactant was cooled to room temperature, The white precipitate was collected on a Buchner funnel, precipitated with cold water several times, obtained crude product of Allantoin. Used 1000 ml distilled water to recrystallize. 60-63% white fine crystalline Allantoin was obtained, melting point was 236 ℃ (decomposition). Calculated by glyoxal, the theoretical yield of Allantoin was 38-40% (weight yield was 103-108%).
Description
Allantoin is a product of purine and uric acid metabolism. It is formed through oxidation of uric acid by urate oxidase in most mammals but is formed only through non-enzymatic oxidation by free radicals in humans. Urinary levels of allantoin are increased prior to the onset of Alzheimer’s disease symptoms in mice expressing mutations in amyloid precursor protein and tau (APP/tau) but not during the early/middle stage of the disease, indicating its potential use as a biomarker for predicting Alzheimer’s disease onset. Due to the formation of allantoin by free radicals in humans, increased urinary levels are a potential biomarker for oxidative stress status.
Chemical Properties
White Solid
Originator
Allantoin ,Arocor Holdings Inc.
Uses
diuretic
Uses
wound healing agent
Uses
Vulnerary; debriding agent. Purine metabolite via the uric acid pathway.
Uses
allantoin is a botanical extract said to be healing, calming, and soothing, it can also help protect the skin from harmful external factors (e.g., wind burn). It is considered an excellent temporary anti-irritant and is believed to stimulate new tissue growth, helping heal damaged skin. Allantoin is appropriate for sensitive, irritated, and acne skins. Derived from the comfrey root, it is considered non-allergenic.
Definition
ChEBI: An imidazolidine-2,4-dione that is 5-aminohydantoin in which a carbamoyl group is attached to the exocyclic nitrogen.
Manufacturing Process
To a mixture of 13.14 kg 40% solution of glyoxal in water and a solution of 0.5 L of concentrated HCl in 3 L of water was added 1.8 g Co(NO3)2·6H2O. The mixture was heated at 50-60°C, to the solution was added 20 g of sodium nitrite and then at 40-60°C was added dropwise the mixture of 6 L of concentrated HNO3, 4.2 L of water and 30 g of sodium nitrite. The product obtained was mixed with 2.4 kg ammonium sulfate and filtrated. The filtrate was heated with 14.5 kg urea at 70°C for 10 hours. Allantoin was filtrated and recrystallized from the water; M.P. 233-235°C.
Therapeutic Function
Vulnerary, Antiulcer (topical), Antipsoriatic
General Description
Allantoin, the final catabolic product of purines in mammals, is a highly polar and amphoteric substance. Allantoin is mainly used for skin protection as the drug promotes cell proliferation and wound healing.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Purine metabolite via the uric acid pathway. Uric acid also reacts with free radicals to produce allantoin, thus allantoin may be a useful biomarker for oxidative stress.
Purification Methods
It crystallises from water or EtOH [Hartman et al. Org Synth Coll Vol II 21 1943]. [Beilstein 25 III/IV 4071.]
Allantoin Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 | admin@china-yime.com | China | 563 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 | sales001@rokechem.com | China | 255 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-199-55145978 +8619955145978 | sales8@anhuiyiao.com | China | 253 | 58 |
| Henan Tengmao Chemical Technology Co. LTD | +8615238638457 | salesvip2@hntmhg.com | China | 415 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 | xinshengkeji@xsmaterial.com | China | 1100 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | sales@zhswyy.com | China | 338 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
View Lastest Price from Allantoin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | allantoin
97-59-6
|
US $25.00 / KG | 25KG | 99% | 1000kg/day | Hebei Yime New Material Technology Co., Ltd. | |
 |
2024-04-25 | Allantoin
97-59-6
|
US $40.00 / kg | 1kg | 99.9% | 200000kg | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-24 | Allantoin
97-59-6
|
US $0.00 / kg | 1kg | 98% | 1000 kg | BINBO BIOLOGICAL CO.,LTD |
97-59-6(Allantoin)Related Search:
1of4