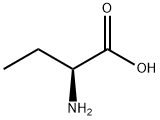
L(+)-2-Aminobutyric acid
- CAS No.
- 1492-24-6
- Chemical Name:
- L(+)-2-Aminobutyric acid
- Synonyms
- AMINOBUTYRIC ACID;L-2-AMINOBUTYRIC ACID;2-AMINOBUTANOIC ACID;L-ABU;H-ABU-OH;L-ALPHA-AMINOBUTYRIC ACID;L-2-AMINOBUTANOIC ACID;L-2-Abu;L-ABU-OH;Z-&gamma
- CBNumber:
- CB3741533
- Molecular Formula:
- C4H9NO2
- Molecular Weight:
- 103.12
- MDL Number:
- MFCD00064415
- MOL File:
- 1492-24-6.mol
- MSDS File:
- SDS
| Melting point | 300 °C |
|---|---|
| Boiling point | 215.2±23.0 °C(Predicted) |
| alpha | 21.6 º (c=2, 5N HCl) |
| Density | 1.2300 (estimate) |
| refractive index | 1.4650 (estimate) |
| storage temp. | room temp |
| solubility | 22.7 g/100 mL (22°C) |
| pka | 2.29(at 25℃) |
| form | Crystalline Powder |
| color | White to beige |
| Water Solubility | 22.7 g/100 mL (22 ºC) |
| Merck | 14,428 |
| BRN | 1720935 |
| InChIKey | QWCKQJZIFLGMSD-VKHMYHEASA-N |
| CAS DataBase Reference | 1492-24-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 0QAJ5KN9IM |
| ATC code | N03AG03 |
| NIST Chemistry Reference | (l)-2-Aminobutanoic acid(1492-24-6) |
| EPA Substance Registry System | Butanoic acid, 2-amino-, (2S)- (1492-24-6) |
L(+)-2-Aminobutyric acid price More Price(59)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A1879 | L-2-Aminobutyric acid ≥99% (titration) | 1492-24-6 | 1g | $120 | 2024-03-01 | Buy |
| Sigma-Aldrich | A1879 | L-2-Aminobutyric acid ≥99% (titration) | 1492-24-6 | 5g | $396 | 2024-03-01 | Buy |
| TCI Chemical | A0826 | (S)-(+)-2-Aminobutyric Acid >99.0%(T) | 1492-24-6 | 1g | $30 | 2024-03-01 | Buy |
| TCI Chemical | A0826 | (S)-(+)-2-Aminobutyric Acid >99.0%(T) | 1492-24-6 | 5g | $84 | 2024-03-01 | Buy |
| Alfa Aesar | J11079 | L-alpha-Amino-N-Butyric acid, 98+%, Affymetrix/USB | 1492-24-6 | 1g | $62.07 | 2021-12-16 | Buy |
L(+)-2-Aminobutyric acid Chemical Properties,Uses,Production
Chemical Properties
White Crystalline Solid
Uses
L-(+)-2-Aminobutyric acid is used in the biosynthesis of nonribosomal peptides. It acts as a receptor antagonist. It is also used as a chiral reagent. Further, it is used in the determination of substrate of glutamyl cysteine acid synthase. In addition to this, it is also utilized as a drug intermediate.
Uses
aminobutyric acid is an amino acid with water-binding properties and possible anti-inflammatory capacities.
Definition
ChEBI: An optically active form of alpha-aminobutyric acid having L-configuration.
Synthesis Reference(s)
Journal of the American Chemical Society, 68, p. 450, 1946 DOI: 10.1021/ja01207a032
General Description
L-2-Aminobutyric acid is synthesized from L-threonine and L-aspartic acid through a ?-transamination reaction. It is an L-alanine analogue with an ethyl side chain.
Biochem/physiol Actions
L-α-Aminobutyric acid (AABA) is an isomer of the non-natural amino acid aminobutyric acid with activity in the γ-glutamyl cycle that regulates glutathione biosynthesis. Recently AABA has been studied as a supplement to in vitro maturation medium (NCSU 23 medium) for culture of oozytes and embryos. This product has been qualified for use in cell culture. AABA is also used as a substitute amino acid for alanine in studies on peptide function.
Purification Methods
Crystallise butyrine from aqueous EtOH, and the melting point depends on heating rate but has m 303o in a sealed tube. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2399 IR: 2401 1961, Beilstein 4 III 1294, 4 IV 2584.]
L(+)-2-Aminobutyric acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jiangxi Chiyan Biopharmaceutical Technology Co.,Ltd. | +86-18616643091 +86-18616643091 | info@rochipharma.com | China | 246 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10991 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | sales@ichemie.com | China | 990 | 58 |
| Sichuan HongRi Pharma-Tech Co.,Ltd | +86-028-64841719 +8617390183901 | daisy@enlaibio.com | China | 1106 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 | admin@86-ss.com | China | 1000 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29798 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18628 | 58 |
| Taizhou Tianhong Biochemistry Technology Co., Ltd. | 0523-86132544 | sales@thbiochem.com | CHINA | 305 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
Related articles
- L(+)-2-Aminobutyric acid: Innovations in Dental Care and Biocatalytic Synthesis
- L(+)-2-Aminobutyric acid aids non-invasive caries removal in dentistry via the Caridex system, benefiting from efficient biosy....
- Jul 17,2024
View Lastest Price from L(+)-2-Aminobutyric acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-20 | L-2-Abu
1492-24-6
|
US $0.00-0.00 / kg | 1kg | 99% | 1T+ | Sichuan HongRi Pharma-Tech Co.,Ltd | |
 |
2024-09-20 | L(+)-2-Aminobutyric acid
1492-24-6
|
US $99.00-66.00 / kg | 0.00100000kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-09-20 | L(+)-2-Aminobutyric acid
1492-24-6
|
US $5.00 / kg | 1kg | ≥99% | 200mt/year | Jinan Finer Chemical Co., Ltd |
-

- L-2-Abu
1492-24-6
- US $0.00-0.00 / kg
- 99%
- Sichuan HongRi Pharma-Tech Co.,Ltd
-

- L(+)-2-Aminobutyric acid
1492-24-6
- US $99.00-66.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- L(+)-2-Aminobutyric acid
1492-24-6
- US $5.00 / kg
- ≥99%
- Jinan Finer Chemical Co., Ltd
1492-24-6(L(+)-2-Aminobutyric acid)Related Search:
1of4