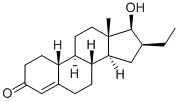
Oxendolone
- CAS No.
- 33765-68-3
- Chemical Name:
- Oxendolone
- Synonyms
- TSAA 291;Roxenone;Prostetin;Oxendolone;ESTR-4-EN-3-ONE;Oxendolone USP/EP/BP;16b-Ethyl-19-nortestosterone;16β-Ethyl-19-nortestosterone;16beta-Ethyl-19-nortestosterone;16β-Ethyl-17β-hydroxyestr-4-en-3-one
- CBNumber:
- CB4969631
- Molecular Formula:
- C20H30O2
- Molecular Weight:
- 302.455
- MDL Number:
- MFCD00864663
- MOL File:
- 33765-68-3.mol
| Melting point | 152-153℃ |
|---|---|
| alpha | D +41° (c = 1.0 in ethanol) |
| Boiling point | 383.47°C (rough estimate) |
| Density | 1.0434 (rough estimate) |
| refractive index | 1.4980 (estimate) |
| pka | 15.08±0.60(Predicted) |
| FDA UNII | MN4I850D4P |
SAFETY
Risk and Safety Statements
| Toxicity | LD50 in rats, mice (g/kg): >10 orally; 5-10 i.m. and i.p. (Hiraga, 1971) |
|---|
Oxendolone price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | API0012147 | OXENDOLONE 95.00% | 33765-68-3 | 5MG | $495.1 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 18733 | Oxendolone | 33765-68-3 | 25mg | $1500 | 2021-12-16 | Buy |
| Crysdot | CD31002191 | Oxendolone 95+% | 33765-68-3 | 1g | $1186 | 2021-12-16 | Buy |
Oxendolone Chemical Properties,Uses,Production
Description
Oxendolone can be used as an anti-androgen drug and has an anti-androgen effect. Competitive antagonism occurs directly with male hormones in the prostate, inhibiting the weight of the prostate and seminal vesicles. The specificity of its anti-androgen effect is high, and it directly competes with androgens in the prostate and hardly shows other hormonal effects.
?Acute toxicity
LD50 in rats and mice (g/kg): >10 orally; 5-10 intramuscular injection and intraperitoneal injection
Originator
Prostetin,Takeda, Japan ,1981
Uses
Oxendolone is used as an anti-androgen (benign prostatic hypertrophy).
Definition
ChEBI: Oxendolone is an organic molecular entity.
Manufacturing Process
To a solution of 3.0 g of 16β-ethylestra-4-ene-3,17-dione dissolved in 150 ml of dioxane, are added 15 g of ethyl orthoformate and 0.1 g of ptoluenesulfonic acid, followed by stirring for 2 hours at room temperature. The reaction solution is poured into 300 ml of a 5% aqueous solution of sodium hydrogen carbonate and the resultant mixture is extracted with ether. The ether layer is washed with water and dried, followed by evaporation of the solvent to give crude crystals of 3-ethoxy-16β-ethylestra-3,5-diene-17-one. The crystals are recrystallized from ether to give 3.0 g of the compound melting at 114°C to 115°C
To a solution of 3.0 g of the enol-ether compound obtained above in 50 ml of methanol, is added 1.5 g of sodium borohydride. After standing for 1.5 hours at room temperature, the reaction solution is poured into 300 ml of water. The resulting precipitates are collected by filtration and recrystallized from ether to give 2.8 g of 3-ethoxy-16β-ethylestra-3,5-dien-17β-ol melting at 131°C to 133°C.
To a solution of 2.5 g of 3-ethoxy-16β-ethylestra-3,5-diene-17β-ol dissolved in
50 ml of methanol is added 1.2 ml of concentrated hydrochloric acid, followed by stirring for 10 minutes. The reaction solution is poured into 250 ml of water. The precipitated crystals are collected by filtration and recrystallized from ether to give 2.3 g of 16β-ethyl-17β-hydroxyestra-4-en-one melting at 152°C to 153°C.
Therapeutic Function
Antiandrogen
Oxendolone Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
Oxendolone Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9320 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29220 | 58 |
| Supplier | Advantage |
|---|---|
| TargetMol Chemicals Inc. | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 58 |
| Dideu Industries Group Limited | 58 |
View Lastest Price from Oxendolone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-06-08 | Oxendolone USP/EP/BP
33765-68-3
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- Oxendolone USP/EP/BP
33765-68-3
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
33765-68-3(Oxendolone)Related Search:
1of4