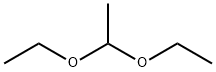
Acetal
- CAS No.
- 105-57-7
- Chemical Name:
- Acetal
- Synonyms
- 1,1-DIETHOXYETHANE;ACETALDEHYDE DIETHYL ACETAL;DIETHYL ACETAL;FEMA 2002;1,1-diethoxy-ethan;1,1-DIETHOXYACETAL;1,1-diethoxyethane acetal;acetal (acetaldehyde diethyl acetal);ACETAL;acetaal
- CBNumber:
- CB5852662
- Molecular Formula:
- C6H14O2
- Molecular Weight:
- 118.17
- MDL Number:
- MFCD00009243
- MOL File:
- 105-57-7.mol
- MSDS File:
- SDS
| Melting point | -100 °C |
|---|---|
| Boiling point | 103 °C |
| Density | 0.831 g/mL at 25 °C(lit.) |
| vapor density | 4.1 (vs air) |
| vapor pressure | 20 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 2002 | ACETAL |
| Flash point | -6 °F |
| storage temp. | Store at +2°C to +8°C. |
| solubility | 46g/l |
| form | Liquid |
| color | Clear colorless |
| Odor | at 1.00 % in propylene glycol. ether green nut earthy sweet vegetable |
| Odor Type | ethereal |
| explosive limit | 1.6-10.4%(V) |
| Water Solubility | 46 g/L (25 ºC) |
| Merck | 14,38 |
| JECFA Number | 941 |
| BRN | 1098310 |
| Dielectric constant | 3.6(21℃) |
| Stability | Stable. Highly flammable. May form peroxides in storage. Test for peroxides before use. Vapors may form an explosive mixture with air, and may travel to source of ignition and flash back. Vapors may spread along ground and collect in low or confined areas (sewers, basements, tanks). |
| InChIKey | DHKHKXVYLBGOIT-UHFFFAOYSA-N |
| LogP | 0.84 |
| Substances Added to Food (formerly EAFUS) | ACETAL |
| FDA 21 CFR | 172.515 |
| CAS DataBase Reference | 105-57-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 5G14F9E2HB |
| NIST Chemistry Reference | Ethane, 1,1-diethoxy-(105-57-7) |
| EPA Substance Registry System | Diethyl acetal (105-57-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H315-H319 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xi | |||||||||
| Risk Statements | 11-36/38 | |||||||||
| Safety Statements | 9-16-33 | |||||||||
| RIDADR | UN 1088 3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | AB2800000 | |||||||||
| Autoignition Temperature | 446 °F &_& 446 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29110000 | |||||||||
| Toxicity | LD50 orally in rats: 4.57 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Acetal price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W200220 | Acetal natural, FG, ≥97% | 105-57-7 | 100g | $93.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | W200220 | Acetal natural, FG, ≥97% | 105-57-7 | 1kg | $391 | 2024-03-01 | Buy |
| Sigma-Aldrich | W200220 | Acetal natural, FG, ≥97% | 105-57-7 | 4kg | $1010 | 2024-03-01 | Buy |
| Sigma-Aldrich | A902 | Acetaldehyde diethyl acetal 99% | 105-57-7 | 5ml | $55.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.01366 | Acetaldehyde diethyl acetal for synthesis | 105-57-7 | 2ml | $26.1 | 2024-03-01 | Buy |
Acetal Chemical Properties,Uses,Production
Synthesis
It can be synthesised by the following steps:A mixture of ketone 236 (50 g, 0.19 mol), 2,2-dimethyl-1,3- propanediol (22.08 g, 0.21 mol) and PPTS (4.85 g, 0.019 mol) was refluxed in benzene in a round bottom flask fitted with Dean-Stark apparatus for 7 hours to remove water. The reaction mixture was washed with aq. NaHCO3 solution, and then thoroughly with water, dried over anhydrous Na2SO4 and filtered. Evaporation of the solvent under reduced pressure followed by crystallization from EtOAc furnished a colourless solid (67.27 g, 95%).
Description
Acetal (full name: Acetaldehyde diethyl acetal/1,1-Diethoxyethane) is a major flavoring component of distilled beverages, especially malt whisky and sherry.
Acetaldehyde diethyl acetal is used as a flavoring agent to provide fruit, nut, rum, and whiskey flavors. It can react with diketene to form ethyl 5-ethoxy-3-oxohexanoate in the presence of titanium chloride. It can also be used to synthesize mixed acetal glycosides via transacetalation.
As a flavor ingredient
Identification:
Synthesis
Acetaldehyde diethyl acetal can be obtained by the reaction between ethyl alcohol and acetaldehyde in the presence of anhydrous calcium chloride.
Preparation
To a pressure bottle containing 20 gm (0.18 mole) of anhydrous calcium chloride is added 105 gm of 95% (2.17 moles) ethanol and the mixture cooled to 8°C. Then 50 gm (1.14 moles) of cold acetaldehyde is slowly poured down the wall of the bottle. The bottle is closed and shaken vigorously for 5-10 min, with cooling if necessary. The mixture is allowed to stand at room temperature with intermittent shaking for 24 hr. The upper layer, which has separated, weighs 128-129 gm. It is washed three times with 30-40 ml of water. The organic layer is dried over 3 gm of anhydrous potassium carbonate and distilled through a 1 ft column, to afford 70-72 gm (59-60%), b.p. 101-103.5°C. The low-boiling fractions are washed again with water, dried and again fractionally distilled to give another 9.0-9.5 gm (7.9-8.1%), b.p. 101-103.5°C. Therefore, the total yield amounts to 79-81.5 gm (67-69%).

References
- Maarse, H. (1991). Volatile Compounds in Foods and Beverages. CRC Press. p. 553. ISBN 0-8247-8390-5.
- Zea, Luis; Serratosa, María P.; Mérida, Julieta; Moyano, Lourdes (2015). "Acetaldehyde as Key Compound for the Authenticity of Sherry Wines: A Study Covering 5 Decades". Comprehensive Reviews in Food Science and Food Safety. 14 (6): 681–693.
Description
Acetal is a clear, colourless, and extremely flammable liquid with an agreeable odour. The vapour is susceptible to cause flash fire. Acetal is sensitive to light and, on storage, may form peroxides. In fact, it has been reported to be susceptible to autoxidation and should, therefore, be classified as peroxidisable. Acetal is incompatible with strong oxidising agents and acids.
Chemical Properties
Acetal.has.a.refreshing,.pleasant,.fruity-green.odor
Chemical Properties
Acetal, an aldehyde, is a clear, volatile liquid with an agreeable odor
Chemical Properties
Acetal is a clear, colorless, and extremely fl ammable liquid with an agreeable odor. The vapor may cause fl ash fi re. Acetal is sensitive to light and on storage may form peroxides. In fact, it has been reported to be susceptible to autoxidation and should, therefore, be classifi ed as peroxidizable. Acetal is incompatible with strong oxidizing agents and acids.
Occurrence
Present.in.some.liquors.(e.g.,.sake,.whiskey.and.cognac);.also.detected.and.quantitatively.assessed.in.rums.. Found.in.apple.juice,.orange.juice,.orange.peel.oil,.bitter.orange.juice,.strawberry.fruit,.raw.radish,.Chinese.quince.fruit,.Chinese. quince.flesh,.udo.(Aralia cordata Thunb.)
Uses
Acetaldehyde diethyl acetal is used as a flavoring agent to provide fruit, nut, rum, and whiskey flavors.
Uses
Solvent; in synthetic perfumes such as jasmine; in organic syntheses.
Preparation
From.ethyl.alcohol.and.acetaldehyde.in.the.presence.of.anhydrous.calcium.chloride.or.small.amounts.of.mineral. acids.(HCl).
Definition
A type of organic compound formed by addition of an alcohol to an aldehyde. Addition of one alcohol molecule gives a hemiacetal. Further addition yields the full acetal. Similar reactions occur with ketones to produce hemiketals and ketals.
Aroma threshold values
Detection:.4.to.42.ppb
General Description
A clear colorless liquid with a pleasant odor. Boiling point 103-104°C. Flash point -5°F. Density 0.831 g / cm3. Slightly soluble in water. Vapors heavier than air. Moderately toxic and narcotc in high concentrations.
Air & Water Reactions
Highly flammable. Forms heat-sensitive explosive peroxides on contact with air. Slightly soluble in water.
Reactivity Profile
Acetal can react vigorously with oxidizing agents. Stable in base but readily decomposed by dilute acids. Forms heat-sensitive explosive peroxides on contact with air. Old samples have been known to explode when heated due to peroxide formation [Sax, 9th ed., 1996, p. 5].
Health Hazard
May irritate the upper respiratory tract. High concentrations act as a central nervous system depressant. Symptoms of exposure include headache, dizziness, drowsiness, abdominal pain, and nausea.
Health Hazard
Mild irritant to skin and eyes; acute toxicityof low order; narcotic at high concentrations;4-hour exposure to 4000 ppm lethal to mice;the oral LD50 value for mice is 3500 mg/kg.
Health Hazard
Exposures to acetal cause irritation to the eyes, skin, gastrointestinal tract, nausea, vomit- ing, and diarrhea. In high concentrations, acetal produces narcotic effects in workers.
Fire Hazard
Highly flammable; flash point (closed cup) -21°C (-6°F); vapor density 4.1 (air = 1), vapor heavier than air and can travel some distance to a source of ignition and flash back; autoignition temperature 230°C (446°F); vapor forms explosive mixtures with air, LEL and UEL values are 1.6% and 10.4% by volume in air, respectively (DOT Label: Flammable Liquid, UN 1088). .
Flammability and Explosibility
Flammable
Industrial uses
Acetal homopolymer resins have high tensilestrength, stiffness, resilience, fatigue endurance,and moderate toughness under repeatedimpact. Some tough grades can deliver up to 7times greater toughness than unmodified acetalin Izod impact tests and up to 30 times greatertoughness as measured by Gardner impact tests.
Automotive applications of acetal homopolymerresins include fuel-system and seat-beltcomponents, steering columns, window-supportbrackets, and handles. Typical plumbingapplications that have replaced brass or zinccomponents are showerheads, ball cocks, faucetcartridges, and various fittings. Consumer itemsinclude quality toys, garden sprayers, stereocassette parts, butane lighter bodies, zippers,and telephone components. Industrial applicationsof acetal homopolymer include couplings,pump impellers, conveyor plates, gears, sprockets,and springs.
Safety Profile
Moderately toxic by ingestion, inhalation, and intraperitoneal routes.A skin and eye irritant. A narcotic. Dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Forms heat-sensitive explosive peroxides on contact with air. when heated to decomposition it emits acrid smoke and fumes. See also ETHERS and ALDEHYDES.
Potential Exposure
Used as a solvent; in synthetic perfumes, such as jasmine, cosmetics, flavors; in organic synthesis.
Metabolism
When acetal was fed at a level of 5% in the diet for 6 days, availability of energy was 64% in chicks and 29% in rats (Yoshida et al. 1970 & 1971). Acetal is rapidly hydrolysed in the stomach(Knoefel, 1934). The resulting acetaldehyde is readily oxidized to acetic acid and eventually to carbon dioxide and water(Williams, 1959).
Shipping
UN1088 Acetal, Hazard Class: 3; Labels: 3-Flammable liquid. UN1988 Aldehydes, flammable, toxic, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous materials, Technical Name Required
Purification Methods
Dry acetal over Na to remove alcohols and H2O, and to polymerise aldehydes, then fractionally distil. Or, treat it with alkaline H2O2 at 40-45o to remove aldehydes, then saturate with NaCl, separate, dry with K2CO3 and distil it from Na [Vogel J Chem Soc 616 1948]. [Beilstein 1 IV 3103.]
Incompatibilities
Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Presumed to form explosive peroxides on contact with air and light. May accumulate static electrical charges, and may cause ignition of its vapors.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Acetal Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
View Lastest Price from Acetal manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-06 | Acetal
105-57-7
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Guanlang Biotechnology Co,.LTD | |
 |
2023-07-27 | Acetal
105-57-7
|
US $1.10 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd | |
 |
2023-02-13 | 1,1-Diethoxyethane
105-57-7
|
US $80.00 / kg | 1kg | 99% | 100MT | Hebei baicao biology science and technology co., ltd |
-

- Acetal
105-57-7
- US $5.00-2.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co,.LTD
-

- Acetal
105-57-7
- US $1.10 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
-

- 1,1-Diethoxyethane
105-57-7
- US $80.00 / kg
- 99%
- Hebei baicao biology science and technology co., ltd
105-57-7(Acetal)Related Search:
1of4