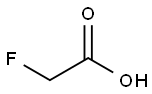
Fluoroacetic acid
- CAS No.
- 144-49-0
- Chemical Name:
- Fluoroacetic acid
- Synonyms
- MFA;HFA;2-Fluoroacetic acid;Cymonic acid;fluoroacetic;2-fluoroaceticacid;Acetic acid, 2-fluoro-;C06108;Un 2642;CH2FCOOH
- CBNumber:
- CB6212623
- Molecular Formula:
- C2H3FO2
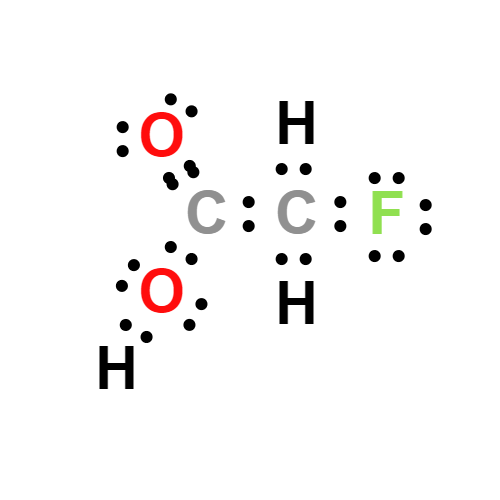
Lewis structure
- Molecular Weight:
- 78.04
- MDL Number:
- MFCD00039529
- MOL File:
- 144-49-0.mol
| Melting point | 33°C |
|---|---|
| Boiling point | 165°C |
| Density | 1.3693 |
| pka | 2.6(at 25℃) |
| Water Solubility | 50mg/L(20 ºC) |
| CAS DataBase Reference | 144-49-0(CAS DataBase Reference) |
| EWG's Food Scores | 4-5 |
| FDA UNII | AP1JV9U41M |
| NIST Chemistry Reference | Acetic acid, fluoro-(144-49-0) |
| EPA Substance Registry System | Fluoroacetic acid (144-49-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300-H400 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P273-P391-P501 |
| Hazard Codes | T+,N |
| Risk Statements | 20/21/22-35-50-28 |
| Safety Statements | 26-36/37/39-45-61-22-20 |
| RIDADR | 2642 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Toxicity | LD50 oral in rat: 4680ug/kg |
Fluoroacetic acid Chemical Properties,Uses,Production
Description
Fluoroacetic acid is a colorless crystallinesolid. Molecular weight=78.05; Boiling point=165C;Freezing/Melting point=35C. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 0, Reactivity 0. Soluble in water.
Chemical Properties
Colorless crystal. Soluble in water and alcohol.
Chemical Properties
Fluoroacetic acid is a colorless crystalline solid.
Uses
Rodenticide.
Uses
Fluoroacetic acid (CH2FCOOH) is very poisonous. It is used to kill rats and mice.
Definition
ChEBI: A haloacetic acid that is acetic acid in which one of the methyl hydrogens is substituted by fluorine.
General Description
A colorless crystalline solid. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Water soluble.
Reactivity Profile
Fluoroacetic acid is a halogenated carboxylic acid derivative. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Fluoroacetic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Hazard
Toxic by ingestion.
Health Hazard
Fluoroacetic acid is very toxic; ingestion of small quantities may cause death.
Fire Hazard
When heated to decomposition, Fluoroacetic acid emits highly toxic fumes of fluorine containing compounds. Some of these materials may burn but none ignite readily. These materials may ignite combustibles (wood, paper, oil, etc.).
Safety Profile
Poison by ingestion, subcutaneous, intraperitoneal, and intravenous routes. Affects the human central nervous system, causing convulsions and ventricular fibrdlation. When heated to decomposition it emits toxic fumes of F and Na2O. See also SODIUM FLUOROACETATE.
Potential Exposure
This material is used as a rodenticide and a drug.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Give large quantities of water and induce vomiting. Do notmake an unconscious person vomit. The symptoms of centralnervous system, cardiac, and renal failure do not becomemanifest until a few hours have passed. Specific treatment isnecessary in case of poisoning with this substance; the appropriate means with instructions must be available.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from oxidizers.
Shipping
UN2642 Fluoroacetic acid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Reacts with reducing agents releasing flammable gas.
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regula tions must be observed.