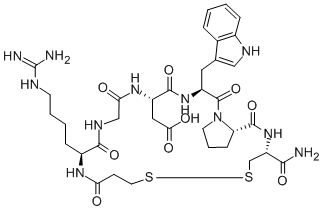
Eptifibatide Acetate
- CAS No.
- 148031-34-9
- Chemical Name:
- Eptifibatide Acetate
- Synonyms
- Eptifitide;INTEGRELIN;Eptifibatide;EFTIFIBATIDE;Human Eptifibatide;EPTIFIBATIDE ACETATE;peptide eptifibatide acetate;Eptifibatide?Acetate impurity;MAP-LYS-GLY-ASP-TRP-PRO-CYS-NH2;Mpr-Har-Gly-Asp-Trp-Pro-Cys-NH2
- CBNumber:
- CB7416565
- Molecular Formula:
- C35H49N11O9S2
- Molecular Weight:
- 831.96
- MDL Number:
- MFCD05662245
- MOL File:
- 148031-34-9.mol
| Melting point | >220°C (dec.) |
|---|---|
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 148031-34-9(CAS DataBase Reference) |
| FDA UNII | NA8320J834 |
| ATC code | B01AC16 |
Eptifibatide Acetate price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Usbiological | 255259 | Eptifibatide | 148031-34-9 | 1mg | $310 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | PEP0001301 | EPTIFIBATIDE ACETATE 95.00% | 148031-34-9 | 1MG | $624.57 | 2021-12-16 | Buy |
| AHH | API-1642 | Eptifibatide 98% | 148031-34-9 | 1g | $1160 | 2021-12-16 | Buy |
Eptifibatide Acetate Chemical Properties,Uses,Production
Pharmacological effects
Eptifibatide is a platelet glycoprotein Ⅱb/Ⅲa receptor reversible antagonist,adverse reactions are mild , it can be discontinued immediately while adverse reactions occur . It has Strong effect and high selectivity . It has no antigenicity,and it does not cause allergic reactions. It is used For acute coronary syndrome, coronary intervention before treatment and acute Q-wave myocardial infarction. It can relieve unstable angina symptoms and reduce the incidence of cardiovascular events. It can limit the non-Q-wave myocardial infarction,and reduce through wall myocardial infarction occurrance.
Eptifibatide is used to treat acute coronary syndrome, the starting amount is 180μg/kg, the intravenous maintenance dose is per minute 2μg/kg continuous intravenous infusion 72h, if implementing the primary percutaneous coronary intervention, continuous intravenous drip after surgery is 18~24h. For percutaneous coronary intervention, the starting amount is 180μg/kg intravenous injection, after 2μg/kg continuous intravenous infusion every minute, after 10 min, administrated again with 180μg/kg intravenously per minute , thereafter 2μg/kg continuous intravenous infusion for 18~24h.
Eptifibatide
Eptifibatide is a cyclic heptapeptide, by preventing fibrinogen, von Willebrand factor and other adhesive ligands binding to GPIIb/IIIa , it can reversibly inhibit platelet aggregation. When administrated intravenously, vitro inhibition of platelet aggregation of eptifibatide is in a dose-and concentration-dependent manner. Inhibition of platelet aggregation is reversible after Eptifibatide infusion is stopped , which is believed to be caused by dissociation of eptifibatide and platelet.
Platelet membrane glycoprotein (GP) Ⅱb/Ⅲa receptor antagonists: after platelet activation, platelet membrane GP Ⅱb/Ⅲa receptor changes its conformation to bind to the end of fibrinogen dimer to complete platelet aggregation. So, GP Ⅱb/Ⅲa receptor is believed to be the final common pathway of platelet aggregation. platelet GP Ⅱb/Ⅲa receptor antagonist Currently in clinical use, it has the following three types; ① abciximab (Abciximab reopro) is the Fab fragment of a monoclonal antibody of platelet GP Ⅱb/Ⅲa receptor. ②Eptifibatide, Integrilin is a cyclic heptapeptide. ③ tirofiban is a small molecule non-peptide compound.
Eptifibatide for injection is a small molecule with heptapeptide containing Lys-Gly-Asp amino acid sequence (KGD), which is like fibrinogen recognizing and binding site GPⅡb/Ⅲa receptors ,it can highly and specially bind to platelets GPⅡb/Ⅲa receptors, it is the GPⅡb/Ⅲa receptor specific competitive inhibitor ,with a low affinity, high dissociation rate, short plasma half-life , platelet aggregation restores baseline levels within 4-8 hours after stopping the infusion . American College of Cardiology (ACC) and the American Heart Association (AHA) guidelines recommend eptifibatide injection can be used for acute coronary syndrome (unstable angina, non-ST-segment elevation myocardial infarction) or supporting through percutaneous coronary intervention (PCI) drug treatment , it is continuous used for 72 hours.
Long-term animal studies on the carcinogenic potential of eptifibatide are not made. the Ames test, mouse lymphoma cells (L 5178Y, TK +/-) forward mutation test, the human lymphocyte chromosome aberration assay, or mouse micronucleus test, have shown that eptifibatide is not genotoxic. continuous infusion for a daily dose of the Eptifibatide reaching 72 mg/kg/day (calculated according to body surface area, it is 4 times of the maximum recommended human daily dose), has no effect on fertility and reproductive capacity of male and female rats.
The above information is edited by the Chemicalbook of Tian Ye.
Uses
The acetate salt form of eptifibatide (E592000), an arginine-glycine-aspartate-mimetic which reversibly binds to platelets to reduce the risk of cardiac ischemic events.
Application
Eptifibatide is a cyclic heptapeptide containing 6 amino acids and 1 mercaptopropionyl (des-amino cysteinyl) residue. An interchain disulfide bridge is formed between the cysteine amide and the mercaptopropionyl moieties. Chemically it is N6-(aminoiminomethyl)-N2-(3-mercapto-1-oxopropyl)-Llysylglycyl-L-α-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide, cyclic (1→6)-disulfide. Eptifibatide binds to the platelet receptor glycoprotein (GP) IIb/IIIa of human platelets and inhibits platelet aggregation.
The eptifibatide peptide is produced by solution-phase peptide synthesis, and is purified by preparative reverse-phase liquid chromatography and lyophilized. The structural formula is:

Indications
Acute Coronary Syndrome (ACS)
INTEGRILIN? is indicated to decrease the rate of a combined endpoint of death or new myocardial infarction (MI) in patients with ACS (unstable angina [UA]/non-ST-elevation myocardial infarction [NSTEMI]), including patients who are to be managed medically and those undergoing percutaneous coronary intervention (PCI).
Percutaneous Coronary Intervention (PCI)
INTEGRILIN is indicated to decrease the rate of a combined endpoint of death, new MI, or need for urgent intervention in patients undergoing PCI, including those undergoing intracoronary stenting.
Dosage forms
Before infusion of INTEGRILIN, the following laboratory tests should be performed to identify pre-existing hemostatic abnormalities: hematocrit or hemoglobin, platelet count, serum creatinine, and PT/aPTT. In patients undergoing PCI, the activated clotting time (ACT) should also be measured.
The activated partial thromboplastin time (aPTT) should be maintained between 50 and 70 seconds unless PCI is to be performed. In patients treated with heparin, bleeding can be minimized by close monitoring of the aPTT and ACT.
Eptifibatide Acetate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Cellmano Biotech Limited | 0551-65326643 18156095617 | info@cellmano.com | China | 999 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Chengdu Youngshe Chemical Co., Ltd. | +8618108235634 | Cecilia@youngshechem.com | China | 2345 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 893 | 58 |
| Alpha Biopharmaceuticals Co., Ltd | +86-411-39042497 +8613921981412 | sales@alphabiopharm.com | China | 886 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
View Lastest Price from Eptifibatide Acetate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-22 | Eptifibatide Acetate
148031-34-9
|
US $0.00 / kg | 2kg | 98% up by HPLC, GMP | 20ton | Sinoway Industrial co., ltd. | |
 |
2024-04-16 | Eptifibatide Acetate
148031-34-9
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2024-03-08 | Eptifibatide Acetate
148031-34-9
|
US $10.00 / kg | 1kg | 99% | 1000kg | Nantong Guangyuan Chemicl Co,Ltd |
-

- Eptifibatide Acetate
148031-34-9
- US $0.00 / kg
- 98% up by HPLC, GMP
- Sinoway Industrial co., ltd.
-

- Eptifibatide Acetate
148031-34-9
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Eptifibatide Acetate
148031-34-9
- US $10.00 / kg
- 99%
- Nantong Guangyuan Chemicl Co,Ltd