Oxytocin
- CAS No.
- 50-56-6
- Chemical Name:
- Oxytocin
- Synonyms
- OT;OXT;pitocin;OXTOCIN;Oxytocin powder;CYS-TYR-ILE-GLN-ASN-CYS-PRO-LEU-GLY-NH2;syntocinon;endopituitrina;pitons;OT-NPI
- CBNumber:
- CB0366966
- Molecular Formula:
- C43H66N12O12S2
- Molecular Weight:
- 1007.19
- MDL Number:
- MFCD00076731
- MOL File:
- 50-56-6.mol
- MSDS File:
- SDS
| Melting point | 192-194°C |
|---|---|
| alpha | D22 -26.2° (c = 0.53) |
| Boiling point | 1533.3±65.0 °C(Predicted) |
| Density | 1.1086 (rough estimate) |
| refractive index | 1.6700 (estimate) |
| storage temp. | 2-8°C |
| solubility | Very soluble in water. It dissolves in dilute solutions of acetic acid and of ethanol (96 per cent). |
| form | lyophilized powder |
| pka | pKa ~6.1(free amino group on Cys) (Occasionally);~10(free phenol on Tyr) (Occasionally) |
| color | White |
| Water Solubility | Soluble in water. |
| Merck | 13,7049 |
| BRN | 3586108 |
| InChIKey | DSZOEVVLZMNAEH-BXUJZNQYSA-N |
| CAS DataBase Reference | 50-56-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 1JQS135EYN |
| NCI Drug Dictionary | Pitocin |
| ATC code | H01BB02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H300-H361 |
| Precautionary statements | P201-P280-P301+P310a-P308+P313-P405-P501a |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | RS7534000 |
| F | 3-8-10-23 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| HS Code | 2937190000 |
| Toxicity | LD50 oral in rat: > 20520ug/kg |
Oxytocin price More Price(50)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | O3251 | Oxytocin ≥97% (HPLC) | 50-56-6 | 500iu | $62.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 900169 | Oxytocin ≥98 atom % D, ≥95% (CP) | 50-56-6 | 1mg | $707 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1491300 | Oxytocin United States Pharmacopeia (USP) Reference Standard | 50-56-6 | 1vial | $553 | 2024-03-01 | Buy |
| Alfa Aesar | J63421 | Oxytocin, 96% | 50-56-6 | 5mg | $203.65 | 2024-03-01 | Buy |
| Alfa Aesar | J63421 | Oxytocin, 96% | 50-56-6 | 25mg | $765.65 | 2024-03-01 | Buy |
Oxytocin Chemical Properties,Uses,Production
Indications and Usage
Oxytocin (OT) is a type of uterine contraction drug and can be derived from the animal posterior pituitary or chemically synthesized.
Oxytocin is a uterine contraction drug that is mostly used in late pregnancy induction and stagnant birth due to weak uterine contractions. Suitable for inducing labor and alleviating pain. Commonly used with ergot preparations to be used in inducing labor, expediting labor, and in uterine bleeding due to weak uterine contractions following birth or still birth. Nose drops can be used to promote lactation.
Mechanisms of Action
Oxytocin does not contain vasopressin and has no pressure-boosting effects. It can be absorbed through oral mucosa, selectively excite smooth uterine muscle, and intensify its contractions. The uterus is most sensitive to oxytocin when in labor (due to increased estrogen secretion), and an immature uterus will not respond to this drug. During early or mid-term pregnancy, the uterus has a relatively low reactivity to oxytocin, which gradually increases during late-stage pregnancy and peaks during labor. Small doses can strengthen the rhythmic contractions of smooth uterine muscles, increase their contractibility, increase their contraction speed, ensure similar contraction characteristics to that of a natural birth, and maintain polarity and symmetry. Thus, it is used clinically to expedite and induce labor. Large doses cause tonic contractions in the uterine muscles, so it is used clinically to burst blood vessels between muscle fibers, prevent postpartum hemorrhage, and ensuring postpartum recovery. It can also promote lactation by causing the breast ducts to contract and expel milk from the breasts, but it cannot increase the lactation amount.
Pharmacokinetics
Ineffective when taken orally, as it can be damaged by digestive fluids, although it can be absorbed through oral mucosa. 1-3 minutes of venous infusion 0.01 IU can induce physiological uterine contractions (Rhythmic, polar, symmetrical) with a short time span, as its half-life is only 2.5-3 minutes. Large doses cause tonic uterine contractions.
Adverse reactions
Oxytocin derived from cow or pig’s pituitary occasionally causes allergic reactions, and infusing too quickly may lead to mild vasodilation and hypotension. Patients who suffer from abruptio placentae, heart disease, or enlarged uterus, are over 35 years old, have a history of cesarean section or uterine muscle tumor removal, or are experiencing a breech birth should use with caution. Using oxytocin while experiencing a sacral block may lead to severe hypertension and even cerebrovascular rupturing. Cannot be injected in the same solution with norepinephrine. Incompatible with hydrolyzed proteins.
Contradictions
Do not use during birth if there are obvious signs of an unsymmetrical head, incorrect fetal position, exposed umbilical cord, prolapse, complete placenta previa, narrow pelvis, or overly intense uterine contractions. Not to be used by patients with overly narrow pelvises, histories of uterine surgery (including C-sections), excessive pains, blocked birth canals, abruptio placentae, or pregnancy poisoning.
Warnings and Precautions
Dosage and infusion speed must be strictly monitored when used to expedite or induce labor to prevent tonic contractions, which can suffocate the fetus or rupture the uterus.
Description
Oxytocin is a peptide hormone containing nine amino acids secreted and synthesized by the paraventricular and supraoptic nuclei of the hypothalamus and stored and released by the neurohypophysis. It acts in females by inducing uterine contractions during parturition and stimulating milk ejection by the mammary glands. No actions in males are known. Synthetic derivatives of oxytocin are used to induce labor and for therapeutic abortions. In recent years the safety of oxytocin has been greatly enhanced by the use of continuous maternal and fetal monitoring and by the use of controlled intravenous infusion of the drug.
Chemical Properties
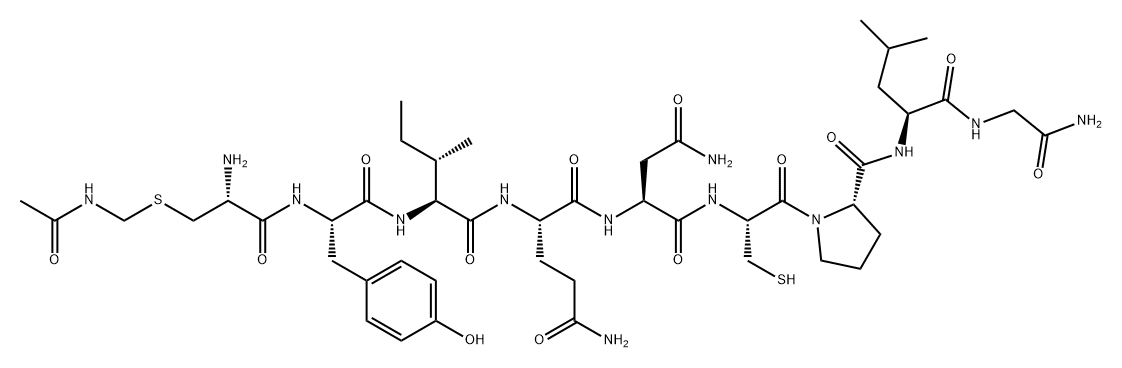
Oxytocin is a white to yellowish brown powder, hygroscopic, easily soluble in water. It is a synthetic cyclic nonapeptide having the structure of the hormone produced by the posterior lobe of the pituitary gland that stimulates contraction of the uterus and milk ejection in receptive mammals.
Originator
Syntocinon,Sandoz,US,1957
Uses
Oxytocin is used as a peptide hormone that acts predominantly as a neurotransmitter; stimulates uterine contraction and lactation, increases Na+ excretion; stimulates myometrial GTPase and phospholipase C.
Uses
Oxytocin is a nonapeptide hormone primarily synthesized in magnocellular neurons of the paraventricular and supraoptic nuclei of the hypothalamus. It is known best for its role in stimulating uterine contraction and lactation and is important for social memory and attachment, sexual and maternal behavior, and aggression. Also, it has been implicated in various non-social behaviors, including learning, anxiety, feeding, and pain perception.
Definition
ChEBI: Oxytocin is a cyclic nonapeptide hormone with amino acid sequence CYIQNCPLG that also acts as a neurotransmitter in the brain; the principal uterine-contracting and milk-ejecting hormone of the posterior pituitary. Together with the neuropeptide vasopressin, it is believed to influence social cognition and behaviour. It has a role as an oxytocic and a vasodilator agent. It is a peptide hormone and a heterodetic cyclic peptide.
brand name
Pitocin (Parkdale); Syntocinon (Novartis);Oxytocin;Pituitrin.
Therapeutic Function
Oxytocic
Biosynthesis
Oxytocin is a cyclic nonapeptide that differs from vasopressin by only 2 amino acids. It is synthesized as a larger precursor molecule in cell bodies of the paraventricular nucleus, and to a lesser extent, the supraoptic nucleus in the hypothalamus. The precursor is rapidly converted by proteolysis to the active hormone and its neurophysin, packaged into secretory granules as an oxytocin-neurophysin complex, and secreted from nerve endings that terminate primarily in the posterior pituitary gland. In addition, oxytocinergic neurons that regulate the autonomic nervous system project to regions of the hypothalamus, brainstem, and spinal cord. Other sites of oxytocin synthesis include the luteal cells of the ovary, the endometrium, and the placenta.
General Description
Oxytocin (OXT) is a potent natriuretic hormone encoded by the gene mapped to human chromosome 20p13. It is synthesized along with its carrier protein neurophysin I from its inactive precursor prepro-OXT. OXT gene consists of three exons and two introns, where first exon codes for hormone OXT while other exon codes for neurophysin I.
Health Hazard
Uterine contraction, milk ejection, facilitates sperm ascent in female tract
Decreases membrane potential of myometrium, basic metabolic rate, and liver glycogen
Stimulates oviposition in hen, releases luteinizing hormone (LH)
Increases blood sugar and urinary sodium and potassium
Biochem/physiol Actions
Oxytocin (OXT) and arginine vasopressin hormone plays a vital role in regulation of water excretion, parturition and lactation. OXT has been implicated in hydromineral homeostasis and vascular and cardiac relaxation. Oxytocin might function as an effective therapeutic for psychiatric diseases, including depression, schizophrenia, anxiety disorders, and autism. OXT has a potential as a marker of autism severity. OXT is an anorexigenic neuropeptide, which is implicated in social cognition and obsessive-compulsive behavior. Plasma oxytocin levels are high in children with Prader-willi syndrome (PWS) compared with unrelated and unaffected siblings. Deficiency of OXT hormone might contribute to pathogenesis of attention deficit/hyperactivity disorder (ADHD).
Clinical Use
Oxytocin is a potent uterine stimulant that is used for the induction and augmentation of labor, antenatal fetal assessment, and control of postpartum hemorrhage. If used improperly, oxytocin can lead to such complications as uterine hypercontractility with fetal distress, uterine rupture, maternal hypotension, water intoxication, and iatrogenic prematurity. These complications can almost always be avoided if oxytocin is given in proper dosages and with careful fetal and maternal monitoring.
Safety Profile
Poison by intravenous route. Experimental reproductive effects. Oxytocin is a pituitary hormone which stimulates uterine contraction and milk production. The principal uterus-contracting and lactation-stimulating hormone of the posterior pituitary gland.
Veterinary Drugs and Treatments
In veterinary medicine, oxytocin has been used for induction or enhancement of uterine contractions at parturition, treatment of postpartum retained placenta and metritis, uterine involution after manual correction of prolapsed uterus in dogs, and in treating agalactia.
storage
-20°C (desiccate)
Purification Methods
It is a cyclic nonapeptide which is purified by countercurrent distribution between solvent and buffer. It is soluble in H2O, n-BuOH and isoBuOH. [Bodanszky & du Vigneaud J Am Chem Soc 81 2504 1959, Cash et al. J Med Pharm Chem 5 413 1962, Sakakibara et al. Bull Chem Soc Jpn 38 120 1965; solid phase synthesis: Bayer & Hagenmyer Tetrahedron Lett 2037 1968.] It was also synthesised on a solid phase matrix and finally purified as follows: A Sephadex G-25 column is equilibrated with the aqueous phase of a mixture of 3.5% AcOH (containing 1.5% of pyridine)/n-BuOH/*C6H6 (2:1:1) and then the organic phase of this mixture is run through. A solution of oxytocin (100mg) in H2O (2mL) is applied to the column which is then eluted with the organic layer of the above mixture. The fractions containing the major peak [as determined by the Folin-Lowry protein assay: Fryer et al. Anal Biochem 153 262 1986] are pooled, diluted with twice their volume of H2O, evaporated to a small volume and lyophilised to give oxytocin as a pure white powder (20mg, 508 U/mg). [Ives Can J Chem 46 2318 1968, Beilstein 22 III/IV 82.]
Oxytocin Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Cellmano Biotech Limited | 0551-65326643 18156095617 | info@cellmano.com | China | 999 | 58 |
| Masteam Bio-tech Co., Ltd. | +86-15200345168 +86-15200345168 | bruce.lei@masteam.com | CHINA | 153 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 878 | 58 |
| Zhuzhou New Peptides Tech Co.,Ltd. | +8618073326374 | sales@goodpeptides.com | China | 140 | 58 |
| Apextide Co Ltd | +undefined15700198315 | senjie.hou@sinopep.com | China | 71 | 58 |
| Henan Tengmao Chemical Technology Co. LTD | +8615238638457 | salesvip2@hntmhg.com | China | 415 | 58 |
| Hebei Xunou new energy Technology Co., LTD | +undefined17531957005 | xunou8@hbxunou.com | China | 36 | 58 |
| Hebei Weimiao Import and Export Trade Co., Ltd. | +undefined19948166995 | sale01@hbweimiao.com | China | 71 | 58 |
| Shanghai Yunao International Trade Co., Ltd | +8617621705551 | asdf@shanghaihg.cn | China | 265 | 58 |
Related articles
- Oxytocin:a hormone and neuropeptide
- Oxytocin, crucial for bonding, also influences stress and social behavior, with medical applications in obstetrics and ongoing....
- Jan 8,2024
- Oxytocin:Mechanism, Application, Adverse Effects, Contraindications and Cautions Studies
- Oxytocin is an oligopeptide hormone that contains nine amino acyl residues, or in other words, a nonapeptide hormone. It is on....
- Feb 21,2023
- Oxytocin
- OT is a mammalian oxytocin family peptide stimulating uterine contraction and milk ejection. Recently, its central effects hav....
- Jun 8,2022
View Lastest Price from Oxytocin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-27 | Oxytocin Acetate
50-56-6
|
US $1.00-9.90 / g | 1g | 99% | 100kg | Wuhan Cell Pharmaceutical Co., Ltd | |
 |
2024-04-26 | Oxytocin
50-56-6
|
US $1.00 / g | 1g | 99% | 100kg | Dorne Chemical Technology co. LTD | |
 |
2024-04-26 | Oxytocin Acetate
50-56-6
|
US $70.00-40.00 / box | 2box | 99% | 100000kg | Hebei Kangcang new material Technology Co., LTD |
-

- Oxytocin Acetate
50-56-6
- US $1.00-9.90 / g
- 99%
- Wuhan Cell Pharmaceutical Co., Ltd
-

- Oxytocin
50-56-6
- US $1.00 / g
- 99%
- Dorne Chemical Technology co. LTD
-

- Oxytocin Acetate
50-56-6
- US $70.00-40.00 / box
- 99%
- Hebei Kangcang new material Technology Co., LTD





