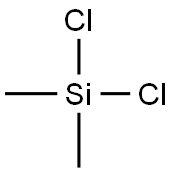Dichlorodimethylsilane
- CAS No.
- 75-78-5
- Chemical Name:
- Dichlorodimethylsilane
- Synonyms
- DIMETHYLDICHLOROSILANE;Me2SiCl2;DIMETHYLSILICON DICHLORIDE;DMDCS;(CH3)2SiCl2;Dichlorodimethylsilicon;ls130;AW-DMCS;Inerton;SILANE M2
- CBNumber:
- CB8854243
- Molecular Formula:
- C2H6Cl2Si
- Molecular Weight:
- 129.06
- MDL Number:
- MFCD00000491
- MOL File:
- 75-78-5.mol
- MSDS File:
- SDS
| Melting point | -76 °C |
|---|---|
| Boiling point | 70 °C(lit.) |
| Density | 1.333 g/mL at 20 °C |
| vapor pressure | <200 hPa (20 °C) |
| refractive index |
n |
| Flash point | 3 °F |
| storage temp. | Store below +30°C. |
| solubility | sol chlorinated solvents and ethereal solvents; reacts with protic solvents. |
| form | liquid |
| Specific Gravity | 1.0637 |
| color | colorless |
| explosive limit | 1.75-48.5%(V) |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 605287 |
| Stability | Stable. Reacts violently with water and alcohols. Highly flammable. Incompatible with strong oxidizing agents, water, alcohols, caustics, ammonia. |
| CAS DataBase Reference | 75-78-5(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 8TSJ92JX69 |
| NIST Chemistry Reference | Silane, dichlorodimethyl-(75-78-5) |
| EPA Substance Registry System | Dimethyldichlorosilane (75-78-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H304-H314-H336-H361d-H373-H412 | |||||||||
| Precautionary statements | P210-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi,F,N,C | |||||||||
| Risk Statements | 20-59-36/37/38-11-67-65-63-48/20-38-20/21-50/53-37-35-20/22-14-34 | |||||||||
| Safety Statements | 60-61-62-36/37-59-45-36/37/39-26-16-7/9-2 | |||||||||
| RIDADR | UN 2924 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | VV3150000 | |||||||||
| F | 3-10-19-21 | |||||||||
| Autoignition Temperature | 425 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29310095 | |||||||||
| Toxicity | LD50 orally in Rabbit: 6056 mg/kg | |||||||||
| NFPA 704 |
|
Dichlorodimethylsilane price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 80430 | Dichlorodimethylsilane produced by Wacker Chemie AG, Burghausen, Germany, ≥99.0% (GC) | 75-78-5 | 500G | $73.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 80430 | Dichlorodimethylsilane produced by Wacker Chemie AG, Burghausen, Germany, ≥99.0% (GC) | 75-78-5 | 5KG | $419 | 2024-03-01 | Buy |
| Sigma-Aldrich | 80430 | Dichlorodimethylsilane produced by Wacker Chemie AG, Burghausen, Germany, ≥99.0% (GC) | 75-78-5 | 25KG | $1430 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03452 | Dichlorodimethylsilane for synthesis | 75-78-5 | 250ML | $48.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03452 | Dichlorodimethylsilane for synthesis | 75-78-5 | 1L | $161 | 2024-03-01 | Buy |
Dichlorodimethylsilane Chemical Properties,Uses,Production
Description
Dichlorodimethylsilane is a tetrahedral, organosilicon compound with the molecular formula Si(CH3)2Cl2. The compound is a colorless liquid at room temperature, has a pungent odor and readily reacts with water to form both cyclic and linear Si-O chains. On an industrial scale, dichlorodimethylsilane is manufactured as the main precursor to polysilane and dimethylsilicone compounds.
History
James Crafts and Charles Friedel are American chemists who first reported organosilicon compounds in 1863 by synthesizing tetraethylsilane from silicon tetrachloride and diethylzinc. Nevertheless, major progress in organosilicon chemistry occurred when Fredrick Kipping and his students reacted tetrachloride with Grignard reagents to produce diorganodichlorosilanes (R2SiCl2), which they used for their experiments. The dpemand for silicones increased in the 1930s as many aircraft companies needed better insulators for sealing materials and electric motors for aircraft engines; therefore, there was need for efficient synthesis of dichlorodimethylsilane.
Preparation
Dichlorodimethylsilane is prepared by passing methyl chloride through a heated tube packed with copper (I) chloride and ground silicon using Cu2O as the catalyst. Methyl chloride is the passed through a reactor to produce dichlorodimethylsilane.
2 CH3Cl + Si → (CH3)2SiCl2
The other products in this reaction apart from dichlorodimethylsilane include CH3)3SiCl, CH3SiHCl2, and CH3SiCl3, which can be separated fromone another by fractional distillation.
Applications
Dichlorodimethylsilane is majorly used in the production of silicones. Moreover, it is utilized in the synthesis of polysilanes, which are the main precursors to silicon carbide. Dichlorodimethylsilane can be used to coat glass to prevent the adsorption of micro-particles.
Description
Dimethyldichlorosilane is a colorless liquidwith sharp, irritating odor. Molecular weight= 129.07;Specific gravity = 1.07 at 25℃; Boiling point = 70.5℃;Melting point=86℃; Vapor pressure= 110 mmHg at20℃; Flash point = 27℃; Autoignition temperature=375℃. Explosive limits: LEL = 3.4%; UEL=9.5℃.Hazard Identification (based on NFPA-704 M Rating
Chemical Properties
Colorless to brown liquid
Physical properties
mp ?76°C; bp 70–71°C; d 1.064 g cm?3.
Uses
Dichlorodimethylsilane is used to prepare a resin bound siloxane with tertiary alcohols and it is also used as a reagent for synthesis of optically active ansa-mettallocene polymerization catalysts. It acts as a precursor to silicone and polysilane compounds. It is also used in the glass coating to protect it from micro particles. It is involved in the preparation of resin bound siloxane with reactivity towards tertiary alcohols.
Uses
Dichlorodimethylsilane can be used as additive for pinacol cyclization; protecting group for diols and
carbonyl compounds;precursor for a wide variety of siliconbased
reagents.
Dichlorodimethylsilane (1) allows clean
pinacol cyclization of a keto aldehyde to occur without competition
from an aldol reaction (eq 1).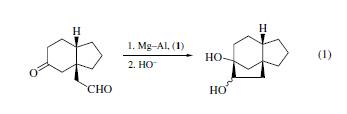
Uses
Dichlorodimethylsilicon is a organosilicon compound and is the precursor to dimethylsilicone and polysilane compounds.
Production Methods
Produced by the action of silicon on methyl chloride in presence of copper catalyst, or by Grignard reaction from methyl chloride and silicon tetrachloride.
General Description
A colorless fuming liquid with a pungent odor. Flash point 16°F. Vapor and liquid may cause burns. Denser than water. Vapors heavier than air.
Reactivity Profile
Chlorosilanes, such as Dichlorodimethylsilane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Health Hazard
Inhalation irritates mucous membranes. Severe gastrointestinal damage may occur. Vapors cause severe eye and lung injury. Upon short contact, second and third degree burns may occur.
Fire Hazard
Vapor may explode if ignited in an enclosed area. Reacts vigorously with water to generate hydrogen chloride. Hydrogen chloride and phosgene gases may be formed upon heating or in fire. Runoff to sewer may create fire or explosion hazard.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by inhalation. A skin and severe eye irritant. Violent reaction on contact with water. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROSILANES.
Potential Exposure
PrimaryIrritant. Used as an intermediate in the manufacture of silicone polymers.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 2448 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
storage
(1) Color Code—Red: Flammability Hazard: Storein a flammable liquid storage area or approved cabinetaway from ignition sources and corrosive and reactivematerials. (2) Color Code—Blue: Health Hazard/Poison:Store in a secure poison location. Dimethyldichlorosilanemust be stored to avoid contact with oxidizers (such as perchlorates, peroxides, permanganates, chlorates, andnitrates), since violent reactions occur. Before entering confined space where this chemical may be present, check tomake sure that an explosive concentration does not exist.Store in tightly closed containers in a cool, well-ventilatedarea away from water, steam, or moisture, because toxicand corrosive hydrogen chloride gas can be produced. Donot store at temperatures above 50℃/122°F. Sources ofignition, such as smoking and open flames, are prohibitedwhere dimethyldichlorosilane is handled, used, or stored.Metal containers involving the transfer of 5 gallons or moreof dimethyldichlorosilane should be grounded and bonded.
Shipping
Dimethyldichlorosilane requires a shipping labelof “FLAMMABLE LIQUID, CORROSIVE.” It falls inHazard Class 3 and Packing Group II
Purification Methods
Other impurities are chlorinated silanes and methylsilanes. Fractionate it through a 3/8in diameter 7ft Stedman column (p 11) rated at 100 theoretical plates at almost total reflux. See purification of MeSiCl2. Solutions in heptane, 1,1,1-trichloroethane or 1-chloronaphthalene are used for the silanization of glassware and pipettes. [Sauer & Hadsell J Am Chem Soc 70 3590 1948, Beilstein 4 IV 4110.]
Incompatibilities
Forms explosive gas mixture with air.Water, steam, and moisture forms toxic and corrosivehydrogen chloride gas. Incompatible with acetone, amines,ammonia, alcohols, strong oxidizers, caustics. Attacks mostmetals. Do not store in temperatures above 122°F/50℃.
Dichlorodimethylsilane Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Qingdao Dexin Chemical Co., Ltd | +8615553333686 | 15553333686@qq.com | China | 926 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Hangzhou Bayee Chemical Co., Ltd. | 0086-571-86990109 | rachelhoo@bayeechem.com | China | 104 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Zjartschem | +86-571-8723 8903 jocelynpan@zjarts.com | jocelynpan@zjarts.com | CHINA | 993 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10248 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
View Lastest Price from Dichlorodimethylsilane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-01-08 | Dichlorodimethylsilane
75-78-5
|
US $6.00 / kg | 1kg | 99.96% | 500ton | Wuhan Boyuan Import & Export Co., LTD | |
 |
2023-08-09 | Dichlorodimethylsilane
75-78-5
|
US $0.00-0.00 / kg | 1kg | 99% | 50tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2023-05-27 | Dichlorodimethylsilane
75-78-5
|
US $100.00 / kg | 1kg | 99.99% | 50000tons | Henan Bao Enluo International TradeCo.,LTD |
-

- Dichlorodimethylsilane
75-78-5
- US $6.00 / kg
- 99.96%
- Wuhan Boyuan Import & Export Co., LTD
-

- Dichlorodimethylsilane
75-78-5
- US $0.00-0.00 / kg
- 99%
- Hebei Yanxi Chemical Co., Ltd.
-

- Dichlorodimethylsilane
75-78-5
- US $100.00 / kg
- 99.99%
- Henan Bao Enluo International TradeCo.,LTD
75-78-5(Dichlorodimethylsilane)Related Search:
1of4