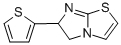Antienite
- CAS No.
- 5029-05-0
- Chemical Name:
- Antienite
- Synonyms
- Antienite;Antienite USP/EP/BP;6-(2-thienyl)-5,6-dihydroimidazo[2,1-b]thiazole;6-thiophen-2-yl-5,6-dihydroimidazo[2,1-b][1,3]thiazole
- CBNumber:
- CB91178044
- Molecular Formula:
- C9H8N2S2
- Molecular Weight:
- 208.308
- MDL Number:
- MOL File:
- 5029-05-0.mol
- MSDS File:
- SDS
| FDA UNII | 50Z1JVP72H |
|---|
Antienite Chemical Properties,Uses,Production
Originator
Antienite,Onbio Inc.
Manufacturing Process
A mixture of 4 parts of 2-iminothiazoline, 8.3 parts of bromomethyl-2-
thienylketone and 40 parts of absolute ethanol is stirred and refluxed for 2
hours in a water-bath. After cooling, the precipitated hydrobromide is filtered
off. From this salt the free base is liberated on treating with ammonium
hydroxide solution and it is extracted with chloroform. The organic extract is
separated, treated with activated charcoal, filtered and the filtrate is first dried
over magnesium sulfate and then evaporated. The solid residue is
recrystallized from 24 parts 2-propanol, to yield 2-imino-3-[(2-
thienylearbonyl)-methyl]-thiazoline; MP: 117.5°-118.5°C.
A mixture of 4 parts of 2-imino-3-[(2-thienylcarbonyl)methyl]thiazoline, 50
parts of acetic anhydride and 1.15 parts of sodium acetate is stirred and
refluxed for 15 minutes. The formed sodium bromide is filtered off. From the
filtrate, the excess of acetic anhydride is distilled and the residual solid is
recrystallized from 2-propanol. The solid is filtered off and dried in vacuum,
yielding 2-(acetylimino)-3-[(2-thienylcarbonyl)methyl]thiazoline; MP: 146°-
147.5°C.
To a stirred mixture of 13 parts of 2-(acetylimino)-3-(2-
thienylcarbonyl)methyl]thiazoline hydrobromide and 64 parts of ethanol are
added portion wise 3 parts of sodium borohydride (exothermic reaction). After
the addition is complete, the whole is stirred and refluxed for one hour. The
solvent is evaporated. The solid residue is dissolved in hydrochloric acid 4 N.
After keeping at room temperature, it crystallizes again. The solid is filtered
off and dissolved in water. The aqueous solution is rendered alkaline with
ammonium hydroxide and extracted with chloroform. The chloroform extract
is dried over magnesium sulfate and evaporated. The solid residue is
recrystallized twice: first from 4-methyl-2-pentanone and once more from 400
parts of water. After drying in vacuum, DLl-2-(acetylimino)-3-[2-hydroxy-2-(2-
thienyl)ethyl]thiazoline is obtained; MP: 132.5°-133°C.
A solution of 2 parts of DL-2-(acetylimino)-3-[2-hydroxy-2-(2-
thienyl)ethyl]thiazoline in 16 parts of thionylchloride and 45 parts chloroform
is stirred and refluxed for one hour. After cooling the whole is extracted with
water. The acid aqueous solution is separated, washed with toluene, alkalized
with ammonium hydroxide solution and extracted with chloroform. The extract
is dried over magnesium sulfate and evaporated. The oily residue is dissolved
in 40 parts boiling 2-propanol. To this warm solution is added a warm solution
of an equivalent quantity of oxalic acid dihydrate in 2-propanoL After cooling
to room temperature, the precipitated oxalate is filtered off and dried in
vacuum, yielding DL-5,6-dihydro-6-(2-thienyl)imidazo[2,1-b]thiazole oxalate;
MP: 192°-193°C.
A mixture of 6 parts DL-2-(acetylimno)-3-[2-hydroxy-2-(2-
thienyl)ethyl]thiazoline and 80 parts phosphoroxy-chloride is heated in a
water-bath for 2 hours at a temperature of 100°C. On cooling, the reaction
mixture is poured into water. The whole is alkalized with ammonium hydroxide
solution and extracted with toluene. The extract is dried over magnesium
sulfate and evaporated in vacuum. The oily residue is dissolved in 40 parts
boiling 2-propanol. To this warm solution is added a warm solution of an
equivalent quantity of oxalic acid dihydrate in 2-propanol. After cooling to
room temperature, the precipitated salt is filtered off and dried in vacuum,
yielding DL-5,6-dihydro-6-(2-thienyl)imidazo[2,1-b]thiazole oxalate; MP:
193°-194°C.
An aqueous solution of this salt is alkalized with ammonium hydroxide and
extracted with toluene. The extract is dried over magnesium sulfate and
evaporated. The oily residue is crystallized from 12 parts xylene. The solid is
filtered off and dried in vacuum, yielding DLl-5,6-dihydro-6-(2-
thienyl)imidazo[2,1-b]thiazole; MP: 58°-62°C.
To a solution of 1.2 parts of DL-5,6-dihydro-6-(2-thienyl)imidazo[2,1-
b]thiazole in 24 parts acetone is added a slight excess of a solution of
hydrogen chloride in 2-propanol. The oily precipitate solidifies on seeding with the solid hydrochloride salt and scratching. The salt is filtered off and dried, to
yield DL-5,6-dihydro-6-(2-thienyl)imidazo[2,1-b]thiazole hydrochloride; M.P.
159°-160.5°C.
Therapeutic Function
Anthelmintic
Antienite Preparation Products And Raw materials
Raw materials
Preparation Products
Antienite Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 29118 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 992 | 58 |
| Supplier | Advantage |
|---|---|
| Dideu Industries Group Limited | 58 |
| Sinoway Industrial co., ltd. | 58 |
View Lastest Price from Antienite manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-07 | Antienite USP/EP/BP
5029-05-0
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- Antienite USP/EP/BP
5029-05-0
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited