フルオキセチン塩酸塩 化学特性,用途語,生産方法
外観
白色~ほとんど白色, 結晶性粉末~粉末
溶解性
メタノール、エタノール、アセトニトリル、クロロホルム、アセトンに可溶。酢酸エチル、水、ジクロロメタンにわずかに可溶。[メタノール85 + 水15(体積比)]に溶ける。
用途
薬理・生理作用研究用。
効能
抗うつ薬, 選択的セロトニン再取り込み阻害薬
説明
Fluoxetine hydrochloride is a selective serotonin reuptake inhibitor (SSRI), it is adapted to the treatment of various depressive disorders, including mild or major depression, bipolar disorder depression, psychogenic depression and depressive neurosis. The mechanism is inhibiting neuronal uptake of serotonin from the synaptic cleft, increasing this neurotransmitter in the gap for practical use , thereby improving the emotional state,and treating depressive disorders. It also appears effective in the treatment of obesity.
化学的特性
White to Off-White Powder
使用
Fluoxetine hydrochloride is a selective serotonin reuptake inhibitor. It is used to treat major depressive disorder, bulimia nervosa (an eating disorder) obsessive-compulsive disorder, panic disorder and premenstrual dysphoric disorder (PMDD).
定義
ChEBI: Fluoxetine hydrochloride (1:1) is a hydrochloride and a N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine. It inhibits the reuptake of serotonin and is used chiefly as an antidepressant.
主な応用
Fluoxetine hydrochloride has been used to study its effect on the binding ability of the radiopharmaceutical 123I-labeled 2-((2-((dimethylamino)methyl)phenyl)thio)-5-iodophenylamine ([123I]ADAM) to SERT (serotonin transporters) in mice. It has also been used for the chronic treatment of light deprived animals.
製造方法
A method for the synthesis of fluoxetine hydrochloride was developed by the Mannich reaction of acetophenone with methylamine hydrochloride, paraformaldehyde to form 3-methylamino-1-phenylacetic acid hydrochloride, followed by the preparation of 3-methylamino-I-phenylpropanol in methanol with potassium borohydride reductant, followed by etherification and salt formation to synthesize the antidepressant fluoxetine monohydrochloride.
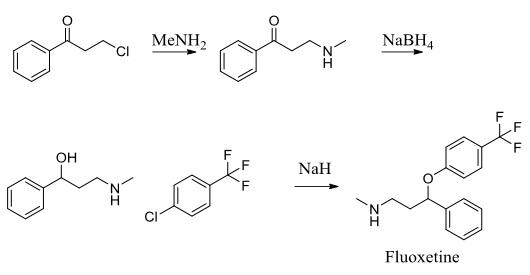
Fluoxetine is the active ingredient in the antidepressant Prozac. It works as a selective serotonin reuptake inhibitor to treat conditions including depression and obsessive-compulsive disorder. To assemble this molecule, a three-step synthesis was utilized. Intermediates included 1- propanone, 3-(methylamino)-1-phenyl-(synthesized through an SN2 reaction between 3-chloropropiophenone and methylamine) and α-[2- (methylamino) ethyl] benzyl alcohol (synthesized through reduction of the first intermediate using NaBH4). The second intermediate was subjected to 4-chlorobenzotrifluoride and sodium hydride to produce the desired molecule, fluoxetine.
一般的な説明
Fluoxetine hydrochloride is an antidepressant drug and a selective serotonin reuptake inhibitor, which is widely used for the treatment of major depressive disorders, obsessive-compulsive disorder and panic fits.
生物活性
Selective serotonin reuptake inhibitor. Binds to the human 5-HT transporter with a K i of 0.9 nmol/l and is between 150- and 900- fold selective over 5-HT 1A , 5-HT 2A , H 1 , α 1 , α 2 -adrenergic, and muscarinic receptors. Antidepressant. Also available as part of the Serotonin Uptake Inhibitor Tocriset™ .
副作用
Common adverse reactions are dry mouth, loss of appetite, nausea, insomnia, fatigue, a few cases of anxiety and headaches are seen . Because fluoxetine hydrochloride has longer half-life, patients whose liver and kidney function are poor or elderly patients, should appropriately reduce the dose. Children should be applied in accordance with the doctor's advice. Patients who has the history of epilepsy, pregnancy or lactation women should use with caution . If rash or fever occurs, the drug should be discontinued immediately and symptomatic treatment should be given. It is not appropriate to be used with monoamine oxidase inhibitor (MAOI) ; if necessary, the drug should be discontinued after five weeks, before switching to a monoamine oxidase inhibitor (MAOI).
フルオキセチン塩酸塩 上流と下流の製品情報
原材料
準備製品