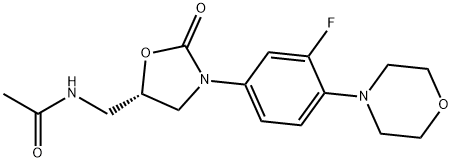
Linezolid
|
|
Linezolid 속성
- 녹는점
- 176-1780C
- 알파
- D20 -9° (c = 0.919 in chloroform)
- 끓는 점
- 585.5±50.0 °C(Predicted)
- 밀도
- 1.302±0.06 g/cm3(Predicted)
- 저장 조건
- room temp
- 용해도
- DMSO: >20mg/mL
- 산도 계수 (pKa)
- 15.53±0.46(Predicted)
- 물리적 상태
- 가루
- 색상
- 흰색에서 황백색까지
- BCS Class
- 4
- InChIKey
- TYZROVQLWOKYKF-ZDUSSCGKSA-N
- CAS 데이터베이스
- 165800-03-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/21/22 | ||
| 안전지침서 | 36-24/25 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AC2720000 | ||
| HS 번호 | 29419000 | ||
| 유해 물질 데이터 | 165800-03-3(Hazardous Substances Data) |
| 그림문자(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | ||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||
| 예방조치문구: |
|
Linezolid C화학적 특성, 용도, 생산
개요
Linezolid reached the US market for the treatment of patients with infections caused by serious Gram-positive pathogens, particularly skin and soft tissue infections, communityacquired pneumonia and vancomycin-resistant enterococcal infections. Linezolid is the (S)-enantiomer of an oxazolidin-2-one synthesized in a multistep process from 3,4- difluoronitrobenzene, the key step being the cyclization of a carbamate, using a chiral epoxyester, into an enantiomerically pure oxazolidin-2-one. Linezolid can be considered as the first of a new class of antibacterial agents known as oxazolidinones, its mechanism of action being related to the inhibition of early ribosomal protein synthesis without directly inhibiting DNA or RNA synthesis. In vitro studies demonstrated that linezolid was effective, at potency levels similar to vancomycin, against staphylococcal, streptococcal and pneumococcal infections (MIC values in the range of 0.5 to 2 μg/ml), enterococcal species including VRE and VSE (MIC values about 4 μg/ml), but also other vancomycin-resistant bacteria. Linezolid is rapidly absorbed orally, its bioavailability is nearly complete at 250 mg dose giving a Cmax to MIC ratio sufficient to have pathogenic strain eradication in the clinical setting. It is considered that this new promising agent may offer new options for therapy of multi-drug infections.화학적 성질
White Solid용도
Linezolid has been used in checkerboard-type drug combination MIC (minimum inhibitory concentrations) assay to determine whether ivacaftor shows positive interaction with linezolid. It has been used for the determination of minimum biofilm inhibitory concentration and for the treatment of Mycobacterium abscessus-infected Drosophila melanogaster w1118 flies.정의
ChEBI: An organofluorine compound that consists of 1,3-oxazolidin-2-one bearing an N-3-fluoro-4-(morpholin-4-yl)phenyl group as well as an acetamidomethyl group at position 5. A synthetic antibacterial agent that inhibits bacterial protein synt esis by binding to a site on 23S ribosomal RNA of the 50S subunit and prevents further formation of a functional 70S initiation complex.원료
Isolates of Staph. aureus and E. faecalis for which the MIC of linezolid is raised have been obtained following serial exposure to gradients of the drug. However, induction of resistance requires many passages over several weeks. Resistance in these laboratory mutants is associated with modifications of the 23S rRNA gene.Overall, resistance rates in clinical isolates are very low at <0.5%. Resistance is reported primarily in coagulase-negative staphylococci (1.77%) and enterococci (1.13%; mostly E. faecium), with exceptionally low resistance rates in Staph. aureus (0.06%). Risk factors for emergence of resistance include prolonged use of the drug, the presence of irremovable indwelling devices, sequestered sites of infection and low-dose therapy for infections caused by vancomycin-resistant enterococci or methicillin-resistant Staph. aureus. Resistance in clinical isolates is most often associated with gene mutations in which guanosine is replaced by uracil in the 23S rRNA. Nosocomial clonal spread of such mutants has been described in coagulase-negative staphylococci and enterococci. Resistance conferred by a novel mobile element, cfr, has been described in two isolates of staphylococci.
일반 설명
Linezolid (Zyvox) is an oxazolidinedione-type antibacterialagent that inhibits bacterial protein synthesis. It acts in theearly translation stage, preventing the formation of a functionalinitiation complex. Linezolid binds to the 30S and 70Sribosomal subunits and prevents initiation complexes involvingthese subunits. Collective data suggest that the oxazolidindionespartition their ribosomal interaction between thetwo subunits. Formation of the early tRNAfMet-mRNA-70Sor 30S is prevented. Linezolid is a newer synthetic agent, andhence, cross-resistance between the antibacterial agent andother inhibitors of bacterial protein synthesis has notbeen seen.Linezolid possesses a wide spectrum of activity againstGram-positive organisms, including MRSA, penicillin-resistantpneumococci, and vancomycin-resistant Enterococcusfaecalis and E. faecium. Anaerobes such as Clostridium,Peptostreptococcus, and Prevotella spp. are sensitive tolinezolid.Linezolid is a bacteriostatic agent against most susceptibleorganisms but displays bactericidal activity against somestrains of pneumococci, B. fragilis, and Clostridiumperfringens.The indications for linezolid are for complicated anduncomplicated skin and soft-tissue infections, communityandhospital-acquired pneumonia, and drug-resistant Grampositiveinfections.
Pharmaceutical Applications
A synthetic oxazolidinone available for oral or intravenous administration. Soluble in water at a pH range of 5–9. Aqueous solutions (2 g/L) are stable at 25°C, 4°C and ?20°C for at least 3 months.생물학적 활성
Oxazolidinone antibiotic. Inhibits bacterial protein synthesis prior to chain initiation. Displays potent antibacterial activity against a variety of multidrug-resistant gram-positive microbes in vitro and in vivo .Mechanism of action
The mechanism of action is inhibition of protein synthesis, but at a stage different from that of other protein synthesis inhibitors. The oxazolidinones bind to the 23S rRNA of the 50S subparticle to prevent formation of a functional 70S initiation complex. It is considered to be bacteriostatic against enterococci and staphylococci but bacteriocidal against streptococci. Resistance to oxazolidinones is encountered in the clinic because of a mutation in the 23S rRNA. This is believed to distort the linezolid binding site. Gram-negative microorganisms are intrinsically resistant to linezolid because of the presence of endogenous efflux pumps that keep it from accumulating in the cells.Clinical Use
Linezolid is primarily used for the treatment of infections caused, or likely to be caused, by methicillin-resistant Staph. aureus, vancomycin-resistant enterococci and penicillin resistant Str. pneumoniae. Combination therapy with an antimicrobial active against Gram-negative bacteria is indicated if concomitant infection with a Gram-negative pathogen is suspected or confirmed.Outside of licensed indications, it has been used in the treatment of bone and joint infections, endocarditis, central nervous system infections, infections in neutropenic patients and drug-resistant tuberculosis.
신진 대사
Linezolid is well absorbed orally and is generally well tolerated; however, some severe cases of reversible blood dyscrasias have been noted, resulting in a package insert warning that complete blood counts should be monitored weekly, especially in patients with poorly draining infections and who are receiving prolonged therapy with the drug. Some interference with monoamine oxidase action has been seen, so patients should be cautious about ingesting tyramine-rich foods. Coadministration with adrenergic and serotonergic agents also is unadvisable. Additionally, lactic acidosis has been reported in patients receiving linezolid. Significant oxidative metabolism of the morpholine ring occurs but is not caused by cytochrome P450, so it does not interfere with other drugs metabolized by this system.Linezolid 준비 용품 및 원자재
원자재
시트르산
부틸리튬
모르폴린
(R)-(-)-3-Chloro-1,2-propanediol
삼차-아밀알코올
벤질 클로로탄소산
염산
4-나이트로벤젠설포닐 염화물
N-BENZYLOXYCARBONYL-3-FLUORO-4-MORPHOLINOANILINE
메탄설포닐 클로라이드
살리실 알데히드
트리에틸아민
N,N-다이아이소프로필에틸아민
Palladium hydroxide
(R)-(-)-글리시딜 부티르산염
소디움 아지이드
포타슘터셔리부톡사이드
준비 용품
Linezolid 공급 업체
글로벌( 802)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Wuhan Xinhao Biotechnology Co., Ltd | +86-18120578002 +86-18120578002 |
xinhao-6@xinhaoshengwu.com | China | 350 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@airuikechemical.com | China | 994 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 |
sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 |
product@chemlin.com.cn | CHINA | 3012 | 60 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ |
sales03@shyrchem.com | CHINA | 738 | 60 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 |
bruce@xrdchem.cn | CHINA | 566 | 55 |
Linezolid 관련 검색:
벤설프론 메틸 티오판산-메틸 디메트모르프 살리실산 메틸 쿠레톡심메틸 (R)-(-)-글리시딜 부티르산염 플루오린(불소) 브롬화 메틸
Voriconazole
Moroxydine
METSULFURON METHYL
Moroxydine hydrochloride
MORPHOLINIUM CHLORIDE
LINEZOLID-D3
Methyl
(S)-5-((Tert-butylamino)methyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one
N,O-Descarbonyl (S)-Linezolid
4-(2-FLUORO-4-NITRO-PHENYL)-MORPHOLINE