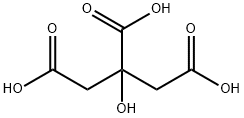
시트르산
|
|
시트르산 속성
- 녹는점
- 153-159 °C (lit.)
- 끓는 점
- 248.08°C (rough estimate)
- 밀도
- 1.67 g/cm3 at 20 °C
- 증기 밀도
- 7.26 (vs air)
- 증기압
- <0.1 hPa (20 °C)
- 굴절률
- 1.493~1.509
- FEMA
- 2306 | CITRIC ACID
- 인화점
- 100 °C
- 저장 조건
- 2-8°C
- 용해도
- 구연산은 또한 15°C에서 무수 에탄올(에탄올 100부당 구연산 76부)에 용해됩니다.
- 물리적 상태
- 모래 형태
- 산도 계수 (pKa)
- 3.14(at 20℃)
- 색상
- 하얀색
- 냄새
- 냄새 없는
- 수소이온지수(pH)
- 3.24(1 mM solution);2.62(10 mM solution);2.08(100 mM solution);
- ?? ??
- 냄새 없는
- 폭발한계
- 8%, 65°F
- 수용성
- 물에 용해됩니다(10°C에서 1174g/L, 30°C에서 1809g/L, 80°C에서 3825g/L).
- 감도
- Hygroscopic
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.20
λ: 280 nm Amax: 0.10
- Merck
- 14,2326
- JECFA Number
- 218
- BRN
- 782061
- 안정성
- 안정적인. 염기, 강산화제, 환원제, 금속 질산염과 호환되지 않습니다.
- InChIKey
- KRKNYBCHXYNGOX-UHFFFAOYSA-N
- LogP
- -1.64
- CAS 데이터베이스
- 77-92-9(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,C,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 41-36/37/38-36/38-37/38-34-36-35-61-60 | ||
| 안전지침서 | 26-39-37/39-24/25-36/37/39-45-36-53 | ||
| 유엔번호(UN No.) | UN 1789 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | GE7350000 | ||
| F 고인화성물질 | 9 | ||
| TSCA | Yes | ||
| HS 번호 | 2918 14 00 | ||
| 유해 물질 데이터 | 77-92-9(Hazardous Substances Data) | ||
| 독성 | LD50 in mice, rats (mmol/kg): 5.0, 4.6 i.p. (Gruber, Halbeisen) | ||
| 기존화학 물질 | KE-20831 |
시트르산 C화학적 특성, 용도, 생산
용도
시트르산은 향료, 킬레이트제, 산성화제로 광범위하게 사용된다. 시트르산은 자연적인 보존제이며, 음식이나 음료수에 산성 또는 신맛을 첨가하기 위해 사용한다. 또한 시트르산은 환경 친화적인 청소제로도 쓰이며, 커피포트 등에 굳은 석회질을 녹이는데도 쓰인다.용도
시트르산은 식품 첨가제로 가장 많은 양이 쓰인다 . 맛과 향을 내거나 식품을 보존시키는 용도로 첨가된다. 아이스크림에 유화제로 첨가되어 지방이 분리되지 않게 하는데 쓰인다. 설탕시럽에 첨가되어 설탕이 석출되지 않게 한다. 신 맛을 내는 용도로 쓰인다. 칼슘과 결합하여 구연산칼슘을 형성하는데 이를 통해 칼슘의 결정화를 막아서 요로결석을 예방하는 효과가 있다. 금속과 결합하여 킬레이트를 형성하는 특성이 있어서, 보일러 등에 낀 산화물 때를 제거하는 데 쓰인다.순도시험
(1) 황산염 : 이 품목 0.5g을 취하여 황산염시험법에 따라 시험할 때, 그 양은 0.01N 황산 0.5mL에 대응하는 양 이하이어야 한다.
(2) 수산염 : 이 품목 1g을 물 10mL에 녹여 염화칼슘시액 2mL를 가할 때, 탁하여서는 아니 된다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 1.3ppm 이하이어야 한다.
(4) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 0.5ppm 이하이어야 한다.
(5) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(6) 칼슘 : 이 품목 1g을 물 10mL에 녹이고 암모니아시액으로 중화한 다음 수산암모늄시액 1mL를 가할 때, 탁하여서는 아니 된다.
(7) 황산정색물 : 이 품목 0.5g에 황산 5mL를 가하고 약 90℃로 1시간 가열하여 녹일 때, 그 액의 색은 비색표준용액 K보다 진하여서는 아니 된다.
(8) 다핵방향족탄화수소 : 이 품목 25g에 물 30mL를 가하여 약 50℃로 가온하여 녹여서 식힌 다음 n-헥산(자외부흡수스펙트럼측정용) 20mL씩 되풀이하여 3회 추출한다. 각각 2,500~3,000rpm에서 약 10분간 원심분리하여 n-헥산층을 합친 다음 n-헥산을 유거하고 1~2mL가 되게 농축하고 식힌 다음 n-헥산(자외부흡수스펙트럼측정용)을 가하여 10mL로 하여 이를 시험용액으로 한다. 시험용액을 액층 1cm에서 흡광도를 측정할 때, 260~350nm의 파장범위내에 있어서는 대조액과의 차가 0.05 이하이어야 한다. 다만, 대조액은 물 30mL에 n-헥산(자외부흡수스펙트럼측정용) 20mL씩 되풀이하여 3회 추출하고 이하 검액과 같이 처리한 액을 쓴다.
(9) 이소구연산 : 이 품목 0.5g을 105℃에서 3시간 가열하고 식힌 다음 아세톤 10mL에 녹이고 그 중 0.005mL를 시험용액으로 하여 여지크로마토그래피 제1법에 따라 시험할 때, 한개의 반점 이외의 다른 반점이 있어서는 아니 된다. 다만, 여지는 크로마토그래피용 2호를 쓴다. 전개용용매가 약 25cm 상승하면 전개를 그치고 풍건한 다음 브로모페놀블루시액(구연산용)을 분무한다. 대조액은 쓰지 아니한다. 또한, 전개용 용매로는 n-부탄올․개미산․물의 혼액(8 : 3 : 2)을 방치한 다음 그 상층을 쓴다.
확인시험
(1) 이 품목의 수용액(1→10)은 산성이다.
(2) 이 품목은 확인시험법 중 구연산염의 반응을 나타낸다.
정량법
이 품목 약 1.5g을 정밀히 달아 물에 녹여 250mL로 하고 그 중 25mL를 취하여 0.1N 수산화나트륨용액으로 적정한다(지시약 : 페놀프탈레인시액 2~3방울).
0.1N 수산화나트륨용액 1mL = 6.404mg C6H8O7
정의
이 품목에는 결정물(일수염) 및 무수물이 있고, 각각을 구연산(결정) 및 구연산(무수)라 칭한다.
강열잔류물
이 품목 2g을 취하여 강열잔류물시험법에 따라 시험할 때, 그 양은 0.05% 이하이어야 한다.
개요
Citric acid is a white, crystalline, weak organic acid present in most plants and many animals as an intermediate in cellular respiration. Citric acid contains three carboxyl groups making it a carboxylic, more specifically a tricarboxylic, acid.the name citrus originates from the Greek kedromelon meaning apple of melon for the fruit citron. Greek works mention kitron, kitrion, or kitreos for citron fruit, which is an oblong fruit several inches long from the scrublike tree Citrus medica. Lemons and limes have high citric acid content, which may account for up to 8% of the fruit's dry weight.
Citric acid is a weak acid and loses hydrogen ions from its three carboxyl groups (COOH) in solution.the loss of a hydrogen ion from each group in the molecule results in the citrate ion,C3H5O(COO)33. A citric acid molecule also forms intermediate ions when one or two hydrogen atoms in the carboxyl groups ionize.the citrate ion combines with metals to form salts, the most common of which is calcium citrate. Citric acid forms esters to produce various citrates, for example trimethyl citrate and triethyl citrate.
화학적 성질
Citric acid is soluble 66 % in water, 33% in alcohol, 3% in ether. Soluble about 20% in Propylene glycol. Virtually odorless. The aqueous solution has a clean acid taste, pleasant in the concentration of 0.02 to 0.08%. Citric acid is a weak organic acid with the formula C6H8O7. It is a natural preservative / conservative and is also used to add an acidic, or sour, taste to foods and soft drinks. In biochemistry, the conjugate base of citric acid, citrate, is important as an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.Citric acid is a commodity chemical, and more than a million tonnes are produced every year by fermentation. It is used mainly as an acidifier, as a flavoring, and as a chelating agent.
물리적 성질
CITRIC ACID, white crystalline solid, decomposes at higher temperatures, sp gr 1.542. Citric acid is soluble in H2O or alcohol and slightly soluble in ether. The compound is a tribasic acid, forming mono-, di-, and tri- series of salts and esters.It occurs in large amounts is citrus fruits, and is used widely in industry as an acidifier, as a flavoring and chelating agent. pKa values are 5.21, 4.28 and 2.92 at 25 °C (extrapolated to zero ionic strength).Citric acid is a good buffering agent for solutions between about pH 2 and pH 8. It is popular in many buffers in many techniques, electrophoresis (SSC Buffer #), to stop reactions, for biopurifications, crystallography... In biological systems around pH 7, the two species present are the citrate ion and mono-hydrogen citrate ion. the pH of a 1 mM solution of citric acid will be about 3.2.
출처
Citric acid exists in greater than trace amounts in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8 % of the dry weight of these fruits (about 47 g/L in the juices ) . The concentrations of citric acid in citrus fruits range from 0.005 mol/L for oranges and grapefruits to 0.30 mol/L in lemons and limes. Within species, these values vary depending on the cultivar and the circumstances in which the fruit was grown.역사
The discovery of citric acid is credited to Jabir ibn Hayyan (Latin name Geber, 721–815).Citric acid was first isolated in 1784 by the Swedish chemist Carl Wilhelm Scheele (1742–1786), who crystallized it from lemon juice.
The crystalline structure of anhydrous citric acid, obtained by cooling hot concentrated solution of the monohydrate form, was first elucidated by Yuill and Bennett in 1934 by X-ray diffraction.
In 1960 Nordman and co-workers further suggested that in the anhydrous form two molecules of the acid are linked through hydrogen bonds between two –COOH groups of each monomer.
용도
Citric Acid is an acidulant and antioxidant produced by mold fermentation of sugar solutions and by extraction from lemon juice, lime juice, and pineapple canning residue. it is the predominant acid in oranges, lemons, and limes. it exists in anhydrous and monohydrate forms. the anhydrous form is crystallized in hot solutions and the monohydrate form is crystallized from cold (below 36.5°c) solutions. anhydrous citric acid has a solubility of 146 g and monohydrate citric acid has a solubility of 175 g/100 ml of distilled water at 20°c. a 1% solution has a ph of 2.3 at 25°c. it is a hygroscopic, strong acid of tart flavor. it is used as an acidulant in fruit drinks and carbonated beverages at 0.25-0.40%, in cheese at 3-4%, and in jellies. it is used as an antioxidant in instant potatoes, wheat chips, and potato sticks, where it prevents spoilage by trapping the metal ions. it is used in combination with antioxidants in the processing of fresh frozen fruits to prevent discoloration.주요 응용
Citric acid is a weak organic acid that is known as a commodity chemical, as more than a million tonnes are produced every year by mycological fermentation on an industrial scale using crude sugar sol utions, such as molasses and strains of Aspergillus niger. Citric acid is widely distributed in plants and in animal tissues and fluids and exist in greater than grace amounts in variety of fruits and vegetables, most notably in citrus fruits such as lemon and limes. Citric acid is mainly used as an acidifier, flavoring agent and chelating agent. It was also used as a chemical restrainer particularly in developers for the collodion process and in silver nitrate solutions used for sensitizing salted and albumen papers.정의
ChEBI: Citric acid is a tricarboxylic acid that is propane-1,2,3-tricarboxylic acid bearing a hydroxy substituent at position 2. It is an important metabolite in the pathway of all aerobic organisms. It has a role as a food acidity regulator, a chelator, an antimicrobial agent and a fundamental metabolite. It is a conjugate acid of a citrate(1-) and a citrate anion.제조 방법
By mycological fermentation using molasses and strains of Aspergillus niger; from citrus juices and pineapple wastes일반 설명
Citric acid appears as colorless, odorless crystals with an acid taste. Denser than water. (USCG, 1999)공기와 물의 반응
The pure material is moisture sensitive (undergoes slow hydrolysis) Water soluble.반응 프로필
Citric acid reacts with oxidizing agents, bases, reducing agents and metal nitrates . Reactions with metal nitrates are potentially explosive. Heating to the point of decomposition causes emission of acrid smoke and fumes [Lewis].건강위험
Inhalation of dust irritates nose and throat. Contact with eyes causes irritation.Biotechnological Applications
Citric acid cycleCitrate, the conjugate base of citric acid is one of a series of compounds involved in the physiological oxidation of fats, proteins, and carbohydrates to carbon dioxide and water.
This series of chemical reactions is central to nearly all metabolic reactions, and is the source of two-thirds of the foodderived energy in higher organisms. Hans Adolf Krebs received the 1953 Nobel Prize in Physiology or Medicine for the discovery. The series of reactions is known by various names, including the "citric acid cycle", the "Krebs cycle" or "Szent-Gy?rgyi — Krebs cycle", and the "tricarboxylic acid (TCA) cycle".
Other biological roles
Citrate is a critical component of bone, helping to regulate the size of calcium crystals.
Safety Profile
Poison by intravenous route. Moderately toxic by subcutaneous and intraperitoneal routes. Mildly toxic byingestion. A severe eye and moderate skin irritant. An irritating organic acid, some allergenic properties. Combustible liquid. Potentially explosive reaction with metal nitrates. When heated to decomposition it emits acrid smoke and fumes.시트르산 준비 용품 및 원자재
원자재
준비 용품
시트르산 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Aurora Industry Co., Ltd. | +86-041139802261 +86-13591747876 |
alex1_auco@126.com | China | 276 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34571 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 |
kyra@quanjinci.com | China | 1534 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 982 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 |
admin@86-ss.com | China | 1000 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 |
admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 |
cooperation@kean-chem.com | China | 40068 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3878 | 58 |
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 |
Ethan@dornechem.com | China | 3138 | 58 |