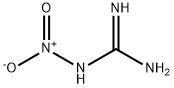
질화구아니딘
|
|
질화구아니딘 속성
- 녹는점
- 239 °C (dec.) (lit.)
- 끓는 점
- 195.15°C (rough estimate)
- 밀도
- 1.55g/cm3
- 증기압
- 0Pa at 20℃
- 굴절률
- 1.6850 (estimate)
- 용해도
- DMSO(약간 용해됨), 메탄올(약간 용해됨, 가열)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- 3.45±0.50(Predicted)
- 색상
- 흰색에서 황백색까지
- 수용성
- 3g/L(20℃)
- Merck
- 14,6607
- BRN
- 1756640
- LogP
- -0.815 at 20℃
- CAS 데이터베이스
- 556-88-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
| 위험품 표기 | F,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-36/37/38-5 | ||
| 안전지침서 | 16-26-33-36/37/39-47-37/39 | ||
| 유엔번호(UN No.) | UN 1336 4.1/PG 1 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | MF4600000 | ||
| 위험 등급 | 1.1D | ||
| 포장분류 | I | ||
| 유해 물질 데이터 | 556-88-7(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-26000 |
질화구아니딘 C화학적 특성, 용도, 생산
화학적 성질
Nitroguanidine is a white powder, It is a well known explosive substance which is used both in high ex plosives and in propellent powders. As a high explosive it is about as powerful as trinitrotoluene and has been used in various mixtures, with ammonium nitrate, paraffin, etc., in bombs and for other purposes where an insensitivir and powerful high explosive is needed. It has the further advantage of be ing exceptionally cool. The experiments of Vieille showed that this explosive produces a temperature of only 907 C. Its coolness commends it for use in safety explosives. If incorporated into nitrocellulose powder it renders the powder flashless without impair ing its ballistic power, and, as Vieille showed, reduces erosion and lengthens the accuracy life of the gun.물리적 성질
Nitroguanidine [556-88-7], CH4N4O2, Mr 104.07, is a very weak base that forms salts only with strong acids. Its solubility in water at 20 ℃ (100 ℃) is 0.27 % (7.6 %). It decomposes at 220 ℃.용도
Nitroguanidine is used in the prediction of heats of detonation of energetic compounds from their molecular structure. It is also used in the synthetic preparation of imidacloprid-d4 (I274992), which is a neonicotinoid.Reactant involved in the synthesis of:
1,5-Disubstituted-1,3,5-hexahydrotriazine-2-(N-nitro)imines with insecticidal activity
Hexahydrotriazine-N-nitroimine analogs with insecticidal activity via Mannich reactions
Reactant involved in:
The study of the effects of vessel materials on exothermic decomposition energy
Nitration of arenes
Nitration of (nitrimino)tetrahyrdooxadiazine and (nitrimino)hexahydrotriazine
일반 설명
Nitroguanidine is shipped as a slurry or wet mass of pale yellow crystals. If Nitroguanidine should dry out Nitroguanidine can explode due to shock, heat, flame, or friction. The primary hazard is blast where the entire load can explode instantaneously and not from flying projectiles and fragments. Under prolonged exposure to fire or heat they can explode.반응 프로필
Nitroalkanes, such as NITROGUANIDINE, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive. Nitroalkanes are insoluble in water. Flammable/combustible material. May be ignited by heat, sparks or flames. DRIED OUT material may explode if exposed to heat, flame, friction or shock; Treat as an explosive. Mercury and silver complex salts of nitroguanidine are very impact sensitive.위험도
May explode when shocked or heated.건강위험
Toxicity of nitroguanidine is very low in test animals. It is nontoxic in rats when given by oral administration at doses as high as 1000 mg/kg/day for 14 days (Morgan et al.1988). The LD50 value, as determined by the oral gavage single-dose limit test, is >5000 mg/kg in both male and female rats (Brown et al. 1988).화재위험
Nitroguanidine is an explosive of moderate power. Its brisance is less than that of TNT or trinitrobenzene. It requires an initiator to explode. On the other hand, nitroguanidine acts as a sensitizer, causing many nonexploding fuel-air systems detonable. Tulis (1984) investigated the detonation of unconfined clouds of aluminum powder in air sensitized with a small amount of nitroguanidine. Aluminum powder dispersed in air becomes more readily detonable when mixed with nitroguanidine. The latter added to a mixture of aluminum powder and potassium chlorate causes transition of the deflagrating mixture into detonable (Tulis et al. 1984). A similarly insensitive explosive, ammonium chlorate, becomes readily detonable when combined with nitroguanidine or nitroguanidine and aluminum powder. Aluminum powder added to a detonable composition of nitroguanidine, and NH4ClO4, caused degradation of the detonation velocity. Moist nitroguanidine containing about 25% water is a flammable solid.Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A very dangerous fire hazard when exposed to heat, flame, or by chemical reaction with oxidizers. A severe explosion hazard when shocked or exposed to heat or flame. It is about as powerful as TNT. It is normally mixed with colloided nitrocellulose or ammonium nitrate and paraffin wax. Can react vigorously with oxidizing materials and the derivatives can be explosive. The mercury and silver salts and other derivatives are much more impact-sensitive. When heated to decomposition it emits highly toxic fumes of NOx. See also NITRO COMPOUNDS.Synthesis
Nitroguanidine is made by treating guanidine nitrate with concentrated sulfuric acid, fuming nitric acid, or both. The reaction of nitroguanidine with amines gives substituted nitroguanidines.Purification Methods
Crystallise it from water (20mL/g). The nitrate has m 147o(dec)( prisms, H2O). [Beilstein 3 H 126, 3 III 236.]질화구아니딘 준비 용품 및 원자재
원자재
준비 용품
질화구아니딘 공급 업체
글로벌( 132)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21666 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8820 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49392 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 |
1015@dideu.com | China | 4088 | 58 |
| CD Chemical Group Limited | +8615986615575 |
info@codchem.com | China | 20356 | 58 |