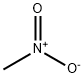
나이트로메테인
|
|
나이트로메테인 속성
- 녹는점
- ?29 °C (lit.)
- 끓는 점
- 101.2 °C (lit.)
- 밀도
- 1.127 g/mL at 25 °C (lit.)
- 증기 밀도
- 2.1 (vs air)
- 증기압
- 27.3 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.382(lit.)
- 인화점
- 95 °F
- 저장 조건
- Flammables area
- 용해도
- 105g/L
- 산도 계수 (pKa)
- 10.2(at 25℃)
- 물리적 상태
- 액체
- 색상
- APHA: ≤10
- 수소이온지수(pH)
- 6.4 (0.6g/l, H2O, 20℃)
- 상대극성
- 6.8
- 냄새
- 불쾌한 과일 냄새
- pH 범위
- 6.4 at 0.01 g/l at 20 °C
- 폭발한계
- 7.3-63.0%(V)
- 수용성
- 9.5g/100mL(20℃)
- 최대 파장(λmax)
- λ: 380 nm Amax: 1.00
λ: 386 nm Amax: 0.50
λ: 395 nm Amax: 0.20
λ: 400 nm Amax: 0.10
λ: 405 nm Amax: 0.05
λ: 430-700 nm Amax: 0.01
- Merck
- 14,6611
- BRN
- 1698205
- Henry's Law Constant
- 2.24 at 20.00 °C, 3.61 at 30.00 °C, 5.40 at 40.00 °C, 7.97 at 50.00 °C (inert gas stripping, Bene? and Dohnal, 1999)
- 노출 한도
- NIOSH REL: IDLH 750 ppm; OSHA PEL: TWA 100 ppm (250 mg/m3); ACGIH TLV: TWA 20 ppm (adopted).
- Dielectric constant
- 22.7(Ambient)
- InChIKey
- LYGJENNIWJXYER-UHFFFAOYSA-N
- CAS 데이터베이스
- 75-52-5(CAS DataBase Reference)
- IARC
- 2B (Vol. 77) 2000
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,F,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 5-10-22 | ||
| 안전지침서 | 41 | ||
| 유엔번호(UN No.) | UN 1261 3/PG 2 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | PA9800000 | ||
| F 고인화성물질 | 3-10 | ||
| 자연 발화 온도 | 784 °F | ||
| 위험 참고 사항 | Irritant/Flammable | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29042090 | ||
| 유해 물질 데이터 | 75-52-5(Hazardous Substances Data) | ||
| 독성 | LC (in air) in guinea pigs: 1000 ppm; LD50 orally in mice: 1.44 g/kg (Weatherby) | ||
| IDLA | 750 ppm | ||
| 기존화학 물질 | KE-26005 | ||
| 사고대비 물질 필터링 | 58 |
나이트로메테인 C화학적 특성, 용도, 생산
물성
아이오딘화메틸과 아질산은을 반응시키면 생긴다. 수용액은 리트머스에 대해 산성을 나타내고, 점화되면 폭발하기 때문에 대량을 취급할 때 알칼리나 그 밖의 이물질(異物質)이 들어가지 않도록 조심해야 한다.용도
살생물제 등의 합성 원료로 쓰인다.용해력이 큰 용매로서 중요하며 로켓의 연료로도 사용된다.용도
코팅산업, 셀룰로스(cellulosic) 화합물, 고분자 & 왁스의 용매, 고분자, 왁스, 연료 첨가제, 로켓 연료용도
"이 제품은 솔벤트, 셀룰로오스 화합물, 폴리머, 수지, 코팅제, 왁스 제품 등에 적용 할 수 있습니다. 또한 점화 마약, 계면 활성제, chloropicrin의 준비를위한 meterial로 사용됩니다. 또한 폭발물, 로켓, 자동차 연료, 레이싱 연료, 의약품, 염료, 살충제 및 휘발유 첨가제를 만들 수 있습니다. "개요
Nitromethane (75-52-5) is an explosive material that was originally manufactured for various applications including mining, construction, demolition, law enforcement, and military uses. However, due to threats of terrorism and increased attention to accident prevention, regulations concerning the transportation, storage, use, and transfer relating to explosives have steadily increased over the last few years and manufacturing limited.화학적 성질
Nitromethane is a highly flammable and explosive colorless liquid with a strong, disagreeable odor. Nitromethane is not explosive, but is used as industrial chemical for various purposes. Nitromethane can explode only in big quantity and in strong confinement. In combination with some further components, nitromethane is the important part of very strong, cap sensitive explosives. Therefore, nitromethane is an easy accessible precursor for preparation of strong home-made explosives.
Nitromethane is used as a stabilizer of halogenated organic solvents, rocket and racing fuel and a chemical intermediate. It is also used as a solvent for cyanoacrylate adhesives, polymers and waxes. It serves as a Michael donor, adding to alfa,beta-unsaturated carbonyl compounds through 1,4-addition in the Michael reaction. It acts as a solvent used for extractions, reaction medium and as a cleaning solvent. Further, it is used in the manufacture of pharmaceuticals, explosives, fibers and coatings.
물리적 성질
Colorless liquid with a strong, disagreeable odor. Odor threshold concentration is 3.5 ppm (quoted, Amoore and Hautala, 1983).용도
Most of the nitromethane produced in the United States (85% to 90%) is used in the synthesis of nitromethane derivatives used as pharmaceuticals, agricultural soil fumigants, and industrial antimicrobials (Markofsky 1991, Angus 2001). Nitromethane also is used as a fuel or fuel additive with methanol in racing cars, boats, and model engines. It formerly was used in the explosives industry as a component in a binary explosive formulation with ammonium nitrate and in shaped charges, and it was used as a chemical stabilizer to prevent decomposition of various halogenated hydrocarbons (NTP 1997, IARC 2000, Angus 2001).생산 방법
Nitromethane and the other important nitroparaffins are synthesized commercially by the vapor-phase nitration of propane (Baker and Bollmeier 1978). At temperatures of 370-450°C and pressures of 8-12 atmospheres, nitromethane, nitroethane and 1- and 2-nitropropane are formed and then separated by distillation.일반 설명
A colorless oily liquid. Flash point 95°F. May violently decompose if intensely heated when contaminated. Denser than water and slightly soluble in water. Hence sinks in water. Vapors are heavier than air. Moderately toxic. Produces toxic oxides of nitrogen during combustion.공기와 물의 반응
Highly flammable. Slightly soluble in water.반응 프로필
Nitromethane may explode if heated or strongly shocked, especially if mixed with acids, bases [Handling Chemicals Safely 1980. p.687], acetone, aluminum powder, ammonium salts in the presence of organic solvents, haloforms (chloroform, bromoform), or hydrazine in methanol. Ignites on contact with alkyl aluminum or alkyl zinc halides. Reacts violently with strong bases (potassium hydroxide, calcium hydroxide), amines (1,2-diaminoethane, hydrazine), bromine, carbon disulfide, hydrocarbons, formaldehyde, metal oxides, lithium aluminum hydride, sodium hydride, strong oxidizing agents (lithium perchlorate, nitric acid, calcium hypochlorite). Reacts with aqueous silver nitrate to form explosive silver fulminate [Bretherick, 5th ed., 1995, p. 183]. Mixtures of Nitromethane and aluminum chloride may explode when organic matter is present [Chem. Eng. News 26:2257. 1948]. Nitromethane, either alone or in a mixture with methanol and castor oil, has a delayed but violent reaction with powdered calcium hypochlorite [Haz. Home Chem 1963]. Nitromethane reacts violently with hexamethylbenzene [Lewis 2544]. Nitromethane is strongly sensitized by hydrazine [Forshey, D. RR. et al, Explosivestoffe, 1969, 17(6), 125-129].위험도
Dangerous fire and explosion risk, lower explosion limit 7.3% in air. Toxic by ingestion and inhalation. Thyroid effects, upper respiratory tract irritant, and lung damage. Possible carcinogen.건강위험
Nitromethane is used primarily as a chemical intermediate in the synthesis of biocides, chemicals, and agricultural products and intermediates. It is slightly toxic to aquatic organisms, has a low bioconcentration potential, and is considered not readily biodegradable. Acute toxicity is low following oral or dermal exposure. Nitromethane is a mild eye irritant and is not likely to cause significant irritation to the skin. Long-term excessive exposure may cause central nervous system effects. Based on animal data, nitromethane is classified as a Category 2B carcinogen (potential human carcinogen).화재위험
Behavior in Fire: Containers may explode공업 용도
Nitromethane is used as an intermediate in chemical syntheses, but more importantly it is used as a solvent for coatings and inks. It and the other nitroparaffins are excellent solvents for vinyls, epoxies, polyamides and acrylic polymers (Baker and Bollmeier 1978). It also is used as a military propellant and a racing fuel additive (HSDB 1988). Mixed with methanol and castor oil it is employed as a model airplane fuel.잠재적 노출
Nitromethane is used in the production of the fumigant, chloropicrin. It is best known as racing car fuel. It is also used as a solvent and as an intermediate in the pharmaceutical industry.Carcinogenicity
Nitromethane is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.환경귀착
Chemical/Physical. Nitromethane will not hydrolyze because it does not contain a hydrolyzable functional group.신진 대사
Nitromethane is converted to nitrite and formaldehyde in a 1:1 ratio by hepatic microsomes from phenobarbital-pretreated male Sprague-Dawley rats (Sakurai et al 1980), but no formaldehyde could be detected when microsomes from the nose or liver of untreated male Fischer-344 rats were incubated with nitromethane (Dahl and Hadley 1983). Whether a similar conversion occurs in vivo has not been determined, but the absence of nitromethane metabolism in microsomes from untreated rats suggests that its metabolism in vivo may be slow.운송 방법
UN1261 Nitromethane, Hazard Class: 3; Labels: 3-Flammable liquid.비 호환성
May explode from heat, shock, friction, or concussion. Reacts with alkalis, strong acids; metallic oxides. Detonates or reacts violently with strong oxidizers, strong reducing agents such as hydrides; formaldehyde, copper, copper alloys; lead, lead alloys; hydrocarbons and other combustibles, causing fire and explosion hazard. Forms shock sensitive mixture when contaminated with acids, amines, bases, metal oxides; hydrocarbons, and other combustible materials.폐기물 처리
Incineration: large quantities of material may require nitrogen oxide removal by catalytic or scrubbing processes.나이트로메테인 준비 용품 및 원자재
원자재
준비 용품
(E)-2-(2-니트로에테닐)티오펜
polythiniren
2,6-Dimethylbenzaldehyde
3-METHOXY-4-PYRIDINECARBOXYLIC ACID
Berberine
2,6-디클로로페네틸이소시아나이드
7-Hydroxy-6-methoxy-3,4-dihydroisoquinoline
티엔펩틴
(E)-2-Nitroethenylbenzene
6-플루오로트립타민 염산염
CYANOMETHYLENETRIBUTYLPHOSPHORANE
3-HYDROXY-4-METHOXYPHENETHYLAMINE HYDROCHLORIDE
4- (2- 아미노 에틸) -2- 메 톡시 페놀
1-니트로메틸사이클로헥사놀
4'-(Trifluoromethoxy)acetophenone
2,3-디메톡시페닐에틸아민
1-니트로프로판
2-니트로프로판
1-AMINOMETHYL-1-CYCLOHEXANOL HYDROCHLORIDE
3-니트로벤조[B]퓨란-5-OL
7-methylisoquinoline
4,5,6,7-Tetrahydrothieno[3,2,c] pyridine hydrochloride
1-(4-하이드록시-3-메톡시페닐)-2-니트로에텐
페놀도팜
파리쿼트(호흡성)
Ticlopidine
2,4-디메톡시페닐에틸아민
2-(3H-이미다졸-4-일)-에틸아민
트리스(하이드록시메틸)니트로메탄
Malotilate
2-플루오로페네틸아민
트리스(하이드록시메틸)아미노메탄
브로노폴
N-Methyl-1-(methylthio)-2-nitroethylen-1-amine
3-히드록시티라민 하이드로브로마이드
DL-Isoserine
이소퀴놀린,7-(브로모메틸)-(9CI)
시클로헵타논
(+/-)-4-AMINO-3-(5-CHLORO-2-THIENYL)-부탄산
1-(아미노메틸)사이클로헥산-1-올
나이트로메테인 관련 검색:
니트로에탄 나이트로벤젠 2-니트로플로렌 나이트로메테인 클로로 피크린(트리클로로니트로메탄) 브로모나이트로메테인 테트라 니트로메탄 트리스(하이드록시메틸)니트로메탄 9-나이트로안트라신 2,7-디니트로-9-플루오레논
NITROCYCLOPENTANE
2-NITROFLUORENONE
2,4,5,7-TETRANITRO-9-FLUORENONE
2,4,7-TRINITRO-9-FLUORENONE
9-DICYANOMETHYLENE-2,4,7-TRINITROFLUORENE
ACID ORANGE 74
2,5-Bis(trifluoromethyl)nitrobenzene
1-NITRO-1-CYCLOHEXENE