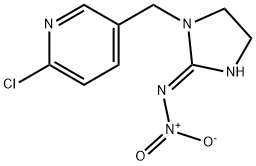
Imidacloprid
|
|
Imidacloprid 속성
- 녹는점
- 144°C
- 끓는 점
- 93.5°C (rough estimate)
- 밀도
- 1.54
- 증기압
- 2 x 10-7
- 굴절률
- 1.5790 (estimate)
- 인화점
- 2 °C
- 저장 조건
- 0-6°C
- 용해도
- DMSO:75.5(Max Conc. mg/mL);295.31(Max Conc. mM)
Ethanol:2.0(Max Conc. mg/mL);7.82(Max Conc. mM)
Water:1.0(Max Conc. mg/mL);3.91(Max Conc. mM)
- 산도 계수 (pKa)
- 7.16±0.20(Predicted)
- 수용성
- 20&C에서 0.061g/100mL
- LogP
- 0.7 at 24℃
- CAS 데이터베이스
- 138261-41-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | N,Xn,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-20/21/22-36-22-20/22 | ||
| 안전지침서 | 26-36-22-36/37-16-46-44 | ||
| 유엔번호(UN No.) | UN 2588 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | NJ0560000 | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | III | ||
| HS 번호 | 29333990 | ||
| 유해 물질 데이터 | 138261-41-3(Hazardous Substances Data) |
Imidacloprid C화학적 특성, 용도, 생산
화학적 성질
Imidacloprid is a white solid crystal or powder under ambient conditions and have a low volatility (U.S. EPA, 2010; HSDB, 2006, 2012b).역사
Imidacloprid was discovered in 1984 at Nihon Bayer Agrochem in Japan by screening novel synthetic compounds for a high affinity to the insect nicotinic AChRs receptors, but with low toxicity to vertebrate species (Kagabu, 1997). Its molecule includes the insecticidal N-(3- pyridinyl)methyl group of nicotine and a nitroimine moiety. Because of their structural similarity to nicotine, imidacloprid and related insecticides (acetamiprid, thiacloprid, thiamethoxam and nitenpyram) were termed neonicotinoids (Tomizawa and Yamamoto, 1993). Nicotine possesses only modest insecticidal activity and is not stable for use in the field for crop protection. Imidacloprid has greater insecticidal activity than nicotine, and its stability is suitable for field use. Both the neonicotinoids and nicotinoids act as agonists at the nAChR. The principal differences between the two classes of compounds are that the nicotinoids are ionized at physiological pH and selective for the mammalian nAChR; whereas the neonicotinoids are not ionized and more selective for the insect nAChR. The selectivity of the neonicotinoids toward insects relative to mammals reflects the fundamental differences in the subunit combination and pharmacological profiles between the nAChR in insects and mammals (for review see Tomizawa and Casida, 2003).용도
Imidacloprid is the active ingredient in AdvantageTM used to control fleas in dogs and cats (HSDB, 2006). Clothianidin is the major metabolite of thiamethoxam and both compounds are registered for use as insecticides. Widespread use increased greatly in the 1990s as alternatives to organophosphosphorus and carbamate insecticides because of their much lower mammalian toxicity and resistance developed to other pesticides. Imidacloprid has become the most widespread insecticide used in the world.
A neonicotinoid; the active ingredient in certain neuro-active insecticides. Reports show that when exposed to neonicotinid pesticides honeybees have probelms returnign home after foraging and bumble bee colonies grow poorly and produce fewer queens.
정의
ChEBI: The E-isomer of imidacloprid.제조 방법
Reduction of 2-chloro-5-pyridinecarbonyl chloride (8) to 2-chloro-5-hydroxymethylpyridine (9) was carried out by excess NaBH4 in water, which was converted to the chloride (10) by SOCl2 . Imidacloprid was obtained by the coupling reaction of 10 with 2-nitroiminoimidazolidine (11) in acetonitrile with potassium carbonate as base. This method was successfully applied to the synthesis of [3H]imidacloprid (12) using NaB[3H]4 as the tritium source (40).Technical production starts with the Tschitschibabin reaction of 3-methylpyridine giving 2-amino-5-methylpyridine (14), which is transformed to the chloride (15) by the Sandmeyer reaction in the presence of hydrogen chloride. A successive operation of chlorination of the methyl group to 10 and the subsequent substitution of the active chloride with ethylene diamine to 16 are carried out without isolation of the intermediates. The final product is produced by ring formation with nitroguanidine. This multistep process affords the product at a purity of >95% (41).
건강위험
Imidacloprid is rapidly and almost completely absorbed (>92%) from the gastrointestinal tract of rats, and is eliminated from the organism rapidly and completely, with no indication of bioaccumulation of the parent compound or its metabolites (WHO, 2001). Similarly, thiamethoxam has relatively low solubility in nonpolar organic solvents and its octanol/water partition coefficient suggests that accumulation in fatty tissues is unlikely to occur (U.S. EPA, 2010).Mechanism of action
Imidacloprid is a neurotoxic insecticide, which belongs to the class of the neonicotinoid pesticides. Imidacloprid is registered to control insect pests on agricultural and nursery crops, structural pests and parasites on companion animals.Imidacloprid is an agonist of the nicotinic acetylcholine receptor (nAChR) at the neuronal and neuromuscular junctions in insects and vertebrates. It is structurally and functionally related to nicotine. The toxicity of imidacloprid is largely due to interference of the neurotransmission in the nicotinic cholinergic nervous system. Prolonged activation of the nAChR by imidacloprid causes desensitization and blocking of the receptor, and leads to incoordination, tremors, decreased activity, reduced body temperature and death. Presently, there is no specific antidote, which acts as an antagonist to the effects imidacloprid.
Pharmacokinetics
Imidacloprid is quickly absorbed by the oral route and rapidly distributed in nearly all organs and tissues. In rats, the oral absorption was estimated as 92-99%. Imidacloprid degrades to a large number of metabolites formed by multiple pathways. The same, or similar metabolites are found in rats, goats and hens. Based on structural considerations, the following metabolites may be of toxicological significance: 6-chloronicotinic acid, imidazolidine 4- and 5- hydroxy compounds, olefinic imidacloprid, desnitro-imidacloprid and the nitrosoimine compound. Metabolites were excreted primarily in the urine as glutathione and glycine conjugates of mercaptonicotinic acid and hippuric acid. Imidacloprid or its metabolites penetrated the blood-brain barrier. The parent compound and some of its metabolites have been detected in milk, meat of goats and hens, and eggs. Pharmacokinetic studies were not available for a direct determination of the rate of absorption from dermal and inhalation routes.Pharmacology
Imidacloprid is a nitroguanidine compound and belongs to the nitromethylene family of chemicals. Themode of action of imidacloprid involves interference with neurological transmission in insects by binding to the postsynaptic nicotinic acetylcholine receptors. Imidacloprid is available as a spot-on treatment for cats and dogs for flea control, and, following application, it distributes throughout the skin within 6 h (Bayer, 1996). It is not absorbed systemically by the animal, and its adulticidal activity is by contact with fleas.Imidacloprid, besides its agricultural use, is also used for the control of subterranean pests and pet ectoparasites.
신진 대사 경로
The photolytic degradation of imidacloprid in different conditions yields diversified degradation products as indicated in the pathways. Photolysis in water gives 6- chloronicotinaldehyde, N-methylnicotinic acid amide, 1- (6-chloro-3-pyridinyl)methyl-2-imidazolinone and 6-chloro-3-pyridylmethylethylenediamine which are identified as main degradates together with a complex mixture of degradation products. On the surface of the tomato plant, four of the 14 metabolites are identified. In tobacco smoke, five degradates are detected, and in field soils, four metabolites are identified from the treated sugarbeet. As for the mammalian metabolites, the pathways are drawn tentatively by the author (see Klein145).신진 대사
Acute oral LD50 for rats: ca. 450 mg/kgImidacloprid 준비 용품 및 원자재
원자재
준비 용품
Imidacloprid 공급 업체
글로벌( 493)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Masteam Bio-tech Co., Ltd. | +86-15200345168 +86-15200345168 |
bruce.lei@masteam.com | CHINA | 153 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@airuikechemical.com | China | 994 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 893 | 58 |
| Hangzhou Bayee Chemical Co., Ltd. | 0086-571-86990109 |
rachelhoo@bayeechem.com | China | 104 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
Imidacloprid 관련 검색:
(E)-N-[(6-클로로-3-피리딜)메틸]-N-시아노-N-메틸아세트아미딘 티아메톡삼 브푸로페진 이미다클로프리드
IMIDACLOPRID D4 (IMIDAZOLIDIN-4,4,5,5 D4)
IMAZALIL D5
Imidacloprid+Fenobucarb,E.C.
Imidacloprid+Validamycin+Monosultap,W.P.
Imidacloprid+Bisultap,dissoluble liquid
Imidacloprid+Thiram+Carboxin,seed coating
Imidacloprid+Triadimefon,W.P.
Imidacloprid+Pyridaben,E.C.
Imidacloprid+Omethoate,E.C.
Imidacloprid+Diesel oil,E.C.
Imidacloprid+Chlorpyrifos,E.C.(22%)
Imidacloprid+Chlorbenzuron,W.P.
Imidacloprid+Profurite-aminium,W.P.
Imidacloprid+Validamycin,W.P.(15%)