카보설판 C화학적 특성, 용도, 생산
개요
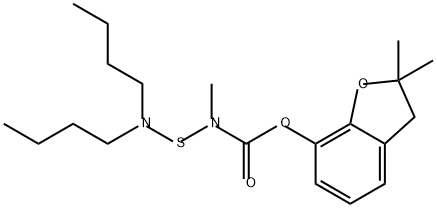
Carbosulfan (8),2,3-dihydro-2,2-dimethylbenzofuran-
7-yl(dibutylaminothio)methylcarbamate(IUPAC),
is an orange to brown, clear viscous liquid (bp 124–128 ?C),
miscible with organic solvents and solubility 0.3 ppm in
water(25 ?C). It is closely related structurally to carbofuran,and like carbofuran, it is a cholinesterase inhibitor with
systemic activity.
화학적 성질
Orange-yellow thick liquid.
용도
Carbosulfan is an insecticide with contact and stomach action. It is
used to control a wide range of soil-dwelling and foliar pests in cotton,
sugar beet, potato, rice, fruit, maize, vegetables, sugar cane and coffee.
일반 설명
Viscous brown liquid.
공기와 물의 반응
Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.
반응 프로필
Carbosulfan is a thiocarbamate. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.
잠재적 노출
Carbosulfan is a carbamate insecticide
and a low toxic derivative from cabofuran. It is a broad
spectrum insecticide, nematicide, miticide, effective against
pests and mites. It is used to protect alfalfa, apple, citrus,
corn, deciduous fruit, potato, rice, sorghum, soybean, sugar
beets, sugarcane, and other vegetable, field, tree and
orchard crops. It is used for seed treatments
신진 대사 경로
Carbosulfan is an N-sulfenyl-N-methylcarbamate which is effectively
a pro-insecticide of carbofuran. The latter is formed in vivo by the
biochemical or chemical thiolysis of carbosulfan. N-S Bond cleavage,
oxidation, conjugation and hydrolysis are the main metabolic routes
for carbosulfan in plants and animals. Carbosulfan is degraded via
carbofuran in soil. In plants, carbosulfan is metabolised via carbofuran to
3-hydroxycarbofuran (PM).
신진 대사
Itsmetabolic patterns are similar to those of carbofuran.
In rats, it rapidly undergoes hydrolytic and oxidative
processes followed by conjugation. It is not persistent in
soils, with DT
50 ca. 2–5 days, and it was rapidly degraded
to carbofuran in a sandy loam soil (4). Carbofuran was
subsequently hydrolyzed at the carbamate ester group
to form the phenol carbofuran or oxidized at the 3-
position. Biscarbofuran disulfide and minor products were
also detected. Carbofuran was also formed in soils by
nonbiological degradation processes.
운송 방법
UN2992 Carbamate pesticides, liquid, toxic,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials;
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1;
Labels: 6.1-Poisonous materials, Technical Name Required.
비 호환성
Carbamates are incompatible with strong
oxidizing acids, peroxides, and hydro-peroxides; strong
reducing agents such as hydrides; strong acids and bases.
Contact with nitrides or chemically active metals (aluminum, copper, magnesium, neptunium, sodium, tin, titanium,zinc, etc.) causes the release of potentially explosive hydrogen gas and a metal salt.
폐기물 처리
Do not discharge into drains
or sewers. Dispose of waste material as hazardous waste
using a licensed disposal contractor to an approved landfill.
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Incineration with
effluent gas scrubbing is recommended. In accordance
with 40CFR165, follow recommendations for the disposal
of pesticides and pesticide containers. Noncombustible containers should be crushed and buried under more than
40 cm of soil. Must be disposed properly by following
package label directions or by contacting your local or federal environmental control agency, or by contacting your
regional EPA office.
카보설판 준비 용품 및 원자재
원자재
준비 용품