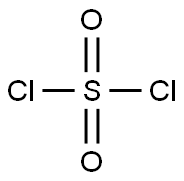염화설퍼릴 C화학적 특성, 용도, 생산
화학적 성질
colourless or pale yellow liquid
물리적 성질
Colorless, mobile liquid; turns yellow on standing; very pungent odor; refractive index 1.4437 at 20°C; density 1.667 g/mL at 20°C; vapors heavier than air, vapor density 4.7 (air=1); melts at -51°C; boils at 69.4°C; sparingly soluble in water, decomposing slowly to sulfuric and hydrochloric acids; forms a hydrate SO
2Cl
2?15H
2O with ice-cold water; miscible with benzene, toluene, chloroform, carbon tetrachloride, and glacial acetic acid; decomposed by alkalies (violent reaction occurs).
용도
Sulfuryl Chloride is a reagent used in the synthesis of catecholic flavonoids as telomerase inhibitors. It is also used to prepare (pyrenebutanamido)thiourea derivs. for anion PET chemosensors.
일반 설명
A colorless fuming liquid with a pungent odor. Very toxic by inhalation. Corrosive to metals and tissue.
반응 프로필
Sulfuryl chloride reacts exothermically with water. Incompatible with strong oxidizing agents, alcohols, amines. Reacts violently with bases. Attacks many metals. Can react explosively with lead dioxide [Mellor 10:676 1946-47]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
위험도
Strong irritant to tissue.
건강위험
Vapors cause severe irritation of eyes and respiratory system. Liquid burns eyes and skin. If ingested, can cause severe burns of mouth and stomach.
화재위험
Behavior in Fire: Toxic and irritating gases are generated.
Safety Profile
A corrosive irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental tumorigenic data. Can explode with Pb02. Will react with water or steam to produce heat and toxic and corrosive fumes. Incompatible with alkalies, diethyl ether, dimethyl sulfoxide,dinitrogen pentaoxide, lead dioxide, phosphorus. When heated to decomposition it emits highly toxic fumes of Cland SOx. See also SULFURIC ACID and HYDROCHLORIC ACID, which are formed upon hydrolysis.
잠재적 노출
Sulfuryl chloride is used to make other chemicals, including chlorophenol and chlorothymol, disinfectants, pharmaceuticals, phosphate insecticides, heterocyclic herbicides, fungicides, dyestuffs, and some plastics; as a solvent and catalyst; as a chlorosulfonating agent in organic synthesis. Also used as a dehydrating agent, as cathode material and in lithium batteries; as a wool treatment to prevent shrinkage
.
운송 방법
UN1834 Sulfuryl chloride, Hazard Class: 6.1; Labels: 6.1-Poison Inhalation Hazard; 8-Corrosive material; Inhalation Hazard Zone A. STN: 4930260; Sulfuryl chloride.
비 호환성
Water and air reactive. When spilled in water, hydrogen chloride, sulfur dioxide and sulfuric acid are produced. Forms corrosive mixture with air (NFPA Fire Rating: 0). Reacts exothermically with moisture in air, water or steam, releasing heat and yielding sulfuric acid and HCl vapors. Reacts violently with bases, amines, amides, inorganic hydroxides; alkalis, alkali metals, dimethyl sulfoxide, dinitrogen pentoxide, lead dioxide (explosive reaction); N-methylformamide, red phosphorus. Reacts, possibly violently, with oxidizers, organic substances, strong acids, alcohols, amines, ethers e.g., diethyl ether, diisopropyl ether, especially if trace amounts of metal salts are present); glycols, peroxides. Attacks metals in the presence of moisture, forming flammable hydrogen gas. Acid formed by reaction with water can be neutralized by limestone, lime, or soda ash.
폐기물 처리
Wear nitrile rubber gloves, laboratory coat, eye protection and, if necessary, a SCBA. Cover the spill with a 1:1:1 mixture by weight of sodium carbonate or calcium carbonate, clay cat litter (bentonite) and sand. When the sulfuryl chloride has been absorbed, scoop the mixture into a plastic pail of cold water. Allow to stand for 24 hours. Test the pH of the solution and neutralize if necessary with sodium carbonate. Decant the solution to the drain flushing with 50 times its volume of water. Treat the solid residue as normal refuse
.
염화설퍼릴 준비 용품 및 원자재
원자재
준비 용품
Disperse Violet 28
2-Chloro-5-chloromethylthiazole
4-클로로-1-에틸-3-메틸-1H-피라졸-5-카르복실산에틸에스테르
Acetazolamide
4-클로로-3-니트로벤젠설포닐클로라이드
6,10,12-트리클로로나프트[2,3-c]아크리딘-5,8,14(13H)-트리온
Pyridine-2-carbonyl chloride hydrochloride
2,3-Dichloropyrazine
Tenoxicam
메빈포스
4-클로로-3-니트로벤젠술폰아미드
클로로넵
DI-N-BUTYL SULFATE
설파마이드
2-ISOCYANATO-1-METHYL-1H-PYRROLE
Tiamenidine
a,a-Dichloro-N,N-Diethylacetylacetamide
DIBUTYL AMIDOSULFENYL CHLORIDE
Lofexidine
아미카티아졸
아세틸(5-)-2,4-디메틸티아졸
2,5-디클로로-3-메틸티오펜
디메틸술파모일 염화물
테브펜피라드
2,5-디클로로티오펜-3-카르보닐클로라이드
1-벤조히오프렌-2-설포닐클로라이드
4-클로로-m-크레졸
N-모르폴리노티오프탈다미드
1-메틸-4-에톡시카르보닐피라졸-5-술포닐염화물
2,3,5-트리클로로티오펜
1,4-디클로로나프탈렌
Seedavay(Uniroyal)
4-사이클로레조시놀
2,5-디클로로벤조-1,4-퀴논
비테르탄올
Disperse purple H-FRL
카복신
2-(사이클로헥실싸이오)-1H-아이소인돌-1,3(2H)-다이온
2-PYRAZINECARBONYL CHLORIDE
2,5-DICHLOROTHIOPHENE-3-CARBOXYLIC ACID