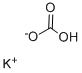
이탄산 칼륨
|
|
이탄산 칼륨 속성
- 녹는점
- 292 °C
- 밀도
- 2,17 g/cm3
- 저장 조건
- Store at +15°C to +25°C.
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 물리적 상태
- 미세한 곡물
- 산도 계수 (pKa)
- 10.33(at 25℃)
- 색상
- 하얀색
- Specific Gravity
- 2.17
- 냄새
- 냄새 없는
- pH 범위
- 8 - 9
- 수소이온지수(pH)
- 8.27(1 mM solution);8.25(10 mM solution);8.13(100 mM solution);
- 수용성
- 물에 용해됩니다. 알코올에 불용성.
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.010
λ: 280 nm Amax: 0.01
- Merck
- 14,7609
- BRN
- 4535309
- 안정성
- 안정적인.
- InChIKey
- TYJJADVDDVDEDZ-UHFFFAOYSA-M
- CAS 데이터베이스
- 298-14-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,T,C,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-35 | ||
| 안전지침서 | 24/25-45-36/37/39-26 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | FG1840000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 28364000 | ||
| 독성 | LD50 orally in Rabbit: > 2000 mg/kg | ||
| 기존화학 물질 | KE-29127 |
이탄산 칼륨 C화학적 특성, 용도, 생산
용 도
1. 탄산 칼륨, 아세트산 칼륨, 아연산 칼륨 및 기타 원료의 생산뿐만 아니라 의학, 식품, 소화 약 및 기타 산업 분야에 사용 2. 일반적으로 분석 시약으로 사용됩니다. 3. 산도 조절기 및 화학 발효제로 사용, 중국은 적절한 사용의 생산 요구에 따라 발효제를 첨가하는 모든 종류의 식품에 사용할 수 있습니다. 4. 탄산 칼륨, 아세트산 칼륨, 비산 칼륨 원료를 생산합니다. 오일 및 화학 물질 소화 약품으로 사용할 수 있습니다.순도시험
(1) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(2.0ppm 이하).
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(3) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(4) 탄산염 : 이 품목 1g을 5℃ 이하의 온도에서 물 20mL를 가하여 흔들어 섞지 않고 녹인 다음 0.1N 염산 2mL와 페놀프탈레인시액 2방울을 가할 때, 엷은 홍색보다 진하여서는 아니 된다.
확인시험
이 품목의 수용액(1→10)은 확인시험법 중 칼륨염 및 탄산수소염의 반응을 나타낸다.
정량법
이 품목 약 4g을 정밀히 달아 물 100mL를 가하여 녹이고 메틸레드시액 2방울을 가하여 계속 저어주면서 엷은 홍색이 될 때까지 1N 염산으로 적정한다. 종말점 부근에서 끓여 식힌 다음 엷은 홍색이 더 이상 엷어지지 않을 때까지 적정을 계속한다.
1N 염산 1mL = 100.1mg KHCO3
화학적 성질
Potassium bicarbonate occurs as colorless, transparent crystals or as a white granular or crystalline powder. It is odorless, with a saline or weakly alkaline taste.용도
Potassium Bicarbonate is an alkali and leavening agent obtained as colorless prisms or white powder. it is very soluble, with 1 g dis- solving in 2.8 ml of water. upon heating, it liberates carbon dioxide which provides leavening in baked goods. it is also used in confec- tionary products.정의
ChEBI: A potassium salt that is the monopotassium salt of carbonic acid. It has fungicidal properties and is used in organic farming for the control of powdery mildew and apple scab.생산 방법
Potassium bicarbonate can be made by passing carbon dioxide into a concentrated solution of potassium carbonate, or by exposing moist potassium carbonate to carbon dioxide, preferably under moderate pressure.Potassium bicarbonate also occurs naturally in the mineral calcinite.
일반 설명
Potassium bicarbonate is water soluble alkaline potassium salt with monoclinic crystalline structure. It is a raw material for the synthesis of many potassium compounds. It is a better coolant than sodium bicarbonate in the aerosol fire extinguishing apparatus. It shows potential as an antifungal agent.농업용
Potassium bicarbonate (KHCO3), also called potassium hydrogen carbonate, is a white crystalline solid, soluble in water (insoluble in ethanol). It decomposes at about 120°C. Potassium bicarbonate contains about 28% potassium (K2O) and used as a potassium supplying fertilizer.Potassium bicarbonate, which occurs naturally as calcinite, is made by passing carbon dioxide into saturated potassium carbonate solution. It is used as baking powder and as a fire extinguisher.
Pharmaceutical Applications
As an excipient, potassium bicarbonate is generally used in formulations as a source of carbon dioxide in effervescent preparations, at concentrations of 25–50% w/w. It is of particular use in formulations where sodium bicarbonate is unsuitable, for example, when the presence of sodium ions in a formulation needs to be limited or is undesirable. Potassium bicarbonate is often formulated with citric acid or tartaric acid in effervescent tablets or granules; on contact with water, carbon dioxide is released through chemical reaction, and the product disintegrates. On occasion, the presence of potassium bicarbonate alone may be sufficient in tablet formulations, as reaction with gastric acid can be sufficient to cause effervescence and product disintegration.Potassium bicarbonate has also been investigated as a gasforming agent in alginate raft systems.The effects of potassium bicarbonate on the stability and dissolution of paracetamol and ibuprofen have been described.
Potassium bicarbonate is also used in food applications as an alkali and a leavening agent, and is a component of baking powder. Therapeutically, potassium bicarbonate is used as an alternative to sodium bicarbonate in the treatment of certain types of metabolic acidosis. It is also used as an antacid to neutralize acid secretions in the gastrointestinal tract and as a potassium supplement.
Safety
Potassium bicarbonate is used in cosmetics, foods, and oral pharmaceutical formulations, where it is generally regarded as a relatively nontoxic and nonirritant material when used as an excipient. However, excessive consumption of potassium bicarbonate or other potassium salts may produce toxic manifestations of hyperkalemia.저장
Potassium bicarbonate should be stored in a well-closed container in a cool, dry location. Potassium bicarbonate is stable in air at normal temperatures, but when heated to 100–200°C in the dry state, or in solution, it is gradually converted to potassium carbonate.Purification Methods
It is crystallised from water at 65-70o (1.25mL/g) by filtering and then cooling to 15o (~0.4ml/g). During all operations, CO2 is passed through the stirred mixture. The crystals are sucked dry at the pump, washed with distilled water, dried in air and then over H2SO4 in an atmosphere of CO2. It is much less soluble than the carbonate in H2O (see below).비 호환성
Potassium bicarbonate reacts with acids and acidic salts with the evolution of carbon dioxide.Regulatory Status
E501 refers to potassium carbonates). Included in nonparenteral medicines licensed in the UK and USA (chewable tablets; effervescent granules; effervescent tablets; lozenges; oral granules; oral suspensions; powder for oral solutions). Included in the Canadian List of Acceptable Non-medicinal Ingredients.이탄산 칼륨 준비 용품 및 원자재
원자재
준비 용품
2-BENZAMIDO-3-(4-(N,N-BIS-(2-클로로에틸)아미노)페닐)프로피온산
아조트리오남
Methyl 7-amino-3-methyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate
LENAMPICILLIN
N-Boc-N'-(2-chlorobenzyloxycarbonyl)-L-lysine
Ethyl 3-bromo-2-(bromomethyl)propionate
4-(4-(BIS-(2-CHLOROETHYL)AMINO)BENZYLIDENE-2-PHENYL-OXAZOLINE-5-ONE
불화붕산 칼륨
H-LYS(2-CL-Z)-OH
CILAZAPRIL
탄산 칼륨
칼륨 메타비아황산염
Cefpiramide acid
타르타르산 칼륨 나트륨
3-FORMYL-AZETIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
칼륨 시트르산, 무수물
(4-메톡시-1H-피롤로[2,3-B]피리딘-2-YL)
폴리덱스트로오스
2-(4-METHOXY-1H-PYRROLO[2,3-B]PYRIDIN-2-YL)ETHANAMINE
알파-[4-(1,1-디메틸에틸)페닐]-4-(하이드록시디페닐메틸)-1-피페리딘부타놀 ; 테르페나딘, 알다반
4-METHOXY-1H-PYRROLO[2,3-B]PYRIDINE-2-CARBALDEHYDE
Sultamicillin
에틸렌4-메톡시-1H-피롤로[2,3-B]피리딘-2-카복실레이트
3-Bromo-2-(bromomethyl)propionic acid
칼륨 벤조산염
이탄산 칼륨 공급 업체
글로벌( 484)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 |
admin@hbandu.com | China | 300 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5882 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 2998 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8820 | 58 |
이탄산 칼륨 관련 검색:
중탄산나트륨(중조) 탄산 칼륨 칼륨 탄산, 1.5수화물 이탄산 칼륨
Sodium Monofluorophosphate
Magnesium carbonate
Ammonium bicarbonate
calcium bicarbonate
MAGNESIUM CARBONATE
potassium ferricyanide
Lead tetraacetate
Sodium carbonate
Vinylene carbonate
Lithium carbonate
Silver carbonate
Cesium carbonate
ChloroMethyl Propyl Carbonate
PRASEODYMIUM CARBONATE