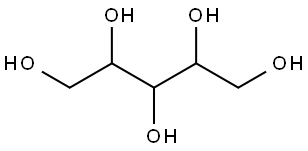
자일리톨
|
|
자일리톨 속성
- 녹는점
- 94-97 °C (lit.)
- 끓는 점
- 215~217℃
- 밀도
- 1.515
- 증기압
- 0.329Pa
- 굴절률
- 1.3920 (estimate)
- 저장 조건
- room temp
- 용해도
- H2O: 0.1 g/mL, 투명, 무색
- 산도 계수 (pKa)
- 13.24±0.20(Predicted)
- 물리적 상태
- 결정성 분말
- 색상
- 하얀색
- 냄새
- 100.00%. 냄새 없는
- 수용성
- 녹는
- 감도
- Hygroscopic
- Merck
- 14,10085
- BRN
- 1720523
- Dielectric constant
- 40.0(Ambient)
- InChIKey
- HEBKCHPVOIAQTA-QWWZWVQMSA-N
- LogP
- -2.56 at 22℃
- CAS 데이터베이스
- 87-99-0(CAS DataBase Reference)
- NIST
- Xylitol(87-99-0)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 24/25-36-26 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | ZF0800000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 29054910 | ||
| 유해 물질 데이터 | 87-99-0(Hazardous Substances Data) | ||
| 독성 | LD50 orally in mice: approx 22 g/kg (Salminen) | ||
| 기존화학 물질 | KE-35438 |
자일리톨 C화학적 특성, 용도, 생산
순도시험
(1) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(2) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(3) 니켈 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(4) 기타 다가알콜류 : 이 품목중의 L-아라비니톨, 갈락티톨, 만니톨, 소비톨의 각 다가알콜함량은 자일리톨정량법과 동일하게 조작하여 다음 계산식에 따라 구한다. 이 때 각 다가알콜의 함량을 모두 합한 값을 구할 때, 그 양은 2% 이하이어야 한다.
WS : 각 다가알콜표준품의 무게(mg)
WU : 검체의 무게(mg)(무수물로서)
RU : 시험용액의 에리스리톨유도체 피크면적에 대한 각 다가알콜유도체 피크면적비
RS : 표준용액중의 에리스리톨유도체 피크면적에 대한 각 다가알콜유도체 피크면적비
(5) 환원당류 : 이 품목 약 500mg을 정밀히 달아 물 2mL가 들어있는 10mL삼각플라스크에 가한 다음 녹인 것을 시험용액으로 한다. 따로 다른 삼각플라스크에 포도당용액(이 액 1mL는 포도당 0.5mg 함유) 2mL를 가해준 다음 각 플라스크에 펠링시액 A액 및 B액을 각각 1mL씩 넣고 끓인 다음 식힐 때, 시험용액은 적갈색의 침전이 생성되는 포도당용액보다 덜 혼탁하여야 한다(0.2% 이하).
확인시험
(1) 이 품목을 적외부흡수스펙트럼측정법의 (1)브롬화칼륨정제법에 따라 시험할 때, 따로 자일리톨표준품을 같은 방법으로 측정하였을 때와 동일한 파장에서만 최대치를 나타내어야 한다. 이 때 만약 차이를 보인다면 이 품목과 표준품을 적당한 용매에 녹여 증발시킨 후 잔류물로서 조작을 반복한다.
(2) 이 품목 5g을 염산․포르말린의 혼액(1 : 1) 10mL에 녹이고 50℃에서 2시간 반응시킨 후 에탄올 25mL를 가한다. 석출된 결정을 여과해서 취하여 이에 물 10mL를 가해 가온하여 용해시키고 에탄올 50mL를 가해서 결정을 석출시킨 후 여과에 의해 분리된 결정을 취한 다음 에탄올을 2회 반복 사용하여 재결정을 하고, 105℃에서 2시간 건조시킨 후 결정의 융점을 측정할 때, 195~201℃이어야 한다.
정량법
이 품목 5g을 정밀히 달아 물에 녹여 100mL로 한 것을 시험원액으로 한다. 따로 자일리톨표준품 4.9g, L-아라비니톨, 갈락티톨, 만니톨 및 소비톨표준품을 각각 25mg씩 정밀히 달아 100mL 메스플라스크에 넣고 이에 물을 가하여 100mL로 한것을 표준원액으로 한다. 시험원액 1mL 및 표준원액 1mL를 100mL 환저플라스크에 각각 취한 후 각 플라스크에 내부표준용액(에리스리톨 500mg을 취하여 물을 가하여 25mL로 한 액) 1mL를 가해주고 감압농축기를 사용하여 60℃의 수욕에서 증발건고시킨 다음 각 건조물에 피리딘 1mL 및 무수초산 1mL를 가해주고 환류냉각기를 부착시켜 수욕상에서 1시간 동안 끓여 완전히 아세틸화 한 액을 각각 시험용액 및 표준용액으로 한다. 시험용액 1μL 및 표준용액 1μL를 각각 가스크로마토그래피에 주입하고 다음 계산식에 따라 자일리톨의 함량(%)을 구한다.
WS : 자일리톨표준품의 무게(mg)
WU : 검체의 무게(mg)(무수물로서)
RU : 시험용액중의 에리스리톨유도체 피크면적에 대한 자일리톨유도체 피크면적비
RS : 표준용액중의 에리스리톨유도체 피크면적에 대한 자일리톨유도체 피크면적비
조작조건
칼 럼 : 내경 2mm, 길이 2m의 유리판 또는 스테인레스관
칼럼충전제 : 크로모소브 W—HP에 3% OV—225를 입힌 것
검 출 기 : 수소염이온화검출기(FID)
주입구온도 : 250℃
칼럼온도 : 200℃
검출기온도 : 250℃
캐리어가스 및 유량 : 질소, 30mL/min
유지시간(Retention time) : 내부표준물질 에리스리톨의 유지시간은 약 3.3분이며, 에리스리톨의 유지시간을 1.0으로 하였을 때, 각 성분들의 상대적인 유지시간은 L-아라비니톨 약 2.77, 자일리톨 약 3.90, 갈락티톨 약 6.96, 만니톨 약 7.63, 소비톨 약 8.43이다.
강열잔류물
이 품목 약 10g을 정밀히 달아 강열잔류물시험법에 따라 시험할 때, 그 양은 0.1% 이하이어야 한다.
화학적 성질
The solubility of D-xylitol (D-xylopentan-1.2.3.4.5-pentaol) in water is approximately 1,690 g/L at room temperature. Xylitol is stable under the common processing conditions of foods.Xylitol is, depending on the concentration, similarly or slightly sweeter than sucrose and noncariogenic.
In the European Union, xylitol is approved as E 967 for a large number of food applications. In the United States, it is approved for use in foods following Good Manufacturing Practice and it is also approved in many other countries.
역사
Xylitol is equally as sweet as sucrose. This property is of advantage to food processors because in reformulating a product from sucrose to xylitol, approximately the same amounts of xylitol can be used. Because xylitol has a negative heat of solution, the substance cools the saliva, producing a perceived sensation of coolness, quite desirable in some food products, notably beverages. Recently, this property has been used in an iced-teaflavored candy distributed in the European market. As of the late 1980s, 28 countries have ruled positively in terms of xylitol for use in commercial products. Xylitol has been found particularly attractive for use in chewing gum, mint and hard candies, and as a coating for pharmaceutical products. Xylitol has the structural formula shown below, with a molecular weight of 152.1. It is a crystalline, white, sweet, odorless powder, soluble in water and slightly soluble in ethanol and methanol. It has no optical activity.용도
A polyol substrate for xylitol and sorbitol dehydrogenases.정의
ChEBI: A pentitol (five-carbon sugar alcohol) having meso-configuration, being derived from xylose by reduction of the carbonyl group.생산 방법
Xylitol occurs naturally in many fruits and berries, although extraction from such sources is not considered to be commercially viable. Industrially, xylitol is most commonly derived from various types of hemicellulose obtained from such sources as wood, corn cobs, cane pulp, seed hulls, and shells. These materials typically contain 20–35% xylan, which is readily converted to xylose (wood sugar) by hydrolysis. This xylose is subsequently converted to xylitol via hydrogenation (reduction). Following the hydrogenation step, there are a number of separation and purification steps that ultimately yield high-purity xylitol crystals. The nature of this process, and the stringent purification procedures employed, result in a finished product with a very low impurity content. Potential impurities that may appear in small quantities are mannitol, sorbitol, galactitol, or arabitol.Less commonly employed methods of xylitol manufacture include the conversion of glucose (dextrose) to xylose followed by hydrogenation to xylitol, and the microbiological conversion of xylose to xylitol.
일반 설명
Xylitol is a naturally occurring five carbon sugar alcohol, equivalent to sucrose in sweetness. Xylitol finds applications in the preparation of confectionaries, chewing gum, toothpaste and mouthwashes. Xylitol is a low-energy sweetener with insulin independent metabolism, making it a promising alternative for sugar in diabetic patients. Xylitol is a natural anticaries agent used in the treatment of dental caries, as it is not utilized by cariogenic bacteria creates a starvation effect on them. Xylitol prevents otitis and upper respiratory tract infections. Commercially, microorganisms like bacteria, fungi and yeasts produce xylitol by fermentation.Safety Profile
Very low toxicity by ingestion. When heated to decomposition it emits acrid smoke and irritating fumes. A sugar.저장
Xylitol is stable to heat but is marginally hygroscopic. Caramelization can occur only if it is heated for several minutes near its boiling point. Crystalline material is stable for at least 3 years if stored at less than 65% relative humidity and 25℃. Milled and specialized granulated grades of xylitol have a tendency to cake and should therefore be used within 9 to 12 months. Aqueous xylitol solutions have been reported to be stable, even on prolonged heating and storage. Since xylitol is not utilized by most microorganisms, products made with xylitol are usually safe from fermentation and microbial spoilage.Xylitol should be stored in a well-closed container in a cool, dry place.
비 호환성
Xylitol is incompatible with oxidizing agents.Regulatory Status
GRAS listed. Approved for use as a food additive in over 70 countries worldwide, including Europe, the USA and Japan. Included in the FDA Inactive Ingredients Database (oral solution, chewing gum). Included in nonparenteral medicines licensed in the UK and USA. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.자일리톨 준비 용품 및 원자재
원자재
준비 용품
자일리톨 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2989 | 55 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 |
hjt@hong-jin.com | China | 228 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3050 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 1143 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18032476855 +86-18032476855 |
admin@hblongbang.com | China | 942 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 |
admin@86-ss.com | China | 1000 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 299 | 55 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5882 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 |
sales06@hbduling.cn | China | 8055 | 58 |
| Across Biotech Jinan Co LTD | +8613031735486 |
frank@acrossbiotech.com | China | 105 | 58 |