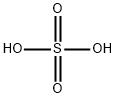
황산
|
|
황산 속성
- 녹는점
- 10°C
- 끓는 점
- ~290 °C (lit.)
- 밀도
- 1.840 g/mL at 25 °C (lit.)
- 증기 밀도
- <0.3 (25 °C, vs air)
- 증기압
- 1 mm Hg ( 146 °C)
- 인화점
- 11 °C
- 저장 조건
- no restrictions.
- 용해도
- H2O: 용해성
- 물리적 상태
- 점성 액체
- 산도 계수 (pKa)
- -3-2(at 25℃)
- 색상
- 연한 노란색에서 약간의 황갈색
- Specific Gravity
- 1.84
- 수소이온지수(pH)
- 2.75(1 mM solution);1.87(10 mM solution);1.01(100 mM solution);
- 냄새
- 냄새 없는
- 수용성
- 혼용 가능
- 감도
- Hygroscopic
- Merck
- 14,8974
- Dielectric constant
- 84.0(20℃)
- 노출 한도
- TLV-TWA air 1 mg/m3 (ACGIH, MSHA, and OSHA); TLV-STEL 3 mg/m3 (ACGIH). .
- 안정성
- Stable, but reacts with moisture very exothermically, which may enhance its ability to act as an oxidizing agent. Substances to be avoided include water, most common metals, organic materials, strong reducing agents, combustible materials, bases, oxidising agents. Reacts violently with water - when diluting concentrated acid, carefully and slo
- LogP
- -1 at 25℃
- CAS 데이터베이스
- 7664-93-9(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,T,F,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/38-35-39/23/24/25-23/24/25-11 | ||
| 안전지침서 | 26-30-45-36/37-16 | ||
| 유엔번호(UN No.) | UN 3264 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | WS5600000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 28070010 | ||
| 유해 물질 데이터 | 7664-93-9(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 2.14 g/kg (Smyth) | ||
| IDLA | 15 mg/m3 | ||
| 기존화학 물질 | KE-32570 | ||
| 유해화학물질 필터링 | 97-1-405 | ||
| 중점관리물질 필터링 | 별표1-127 | ||
| 사고대비 물질 필터링 | 45 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 황산 및 이를 10% 이상 함유한 혼합물 |
황산 C화학적 특성, 용도, 생산
용도
비료용, 황산반토, 염안료 원료용, 폐수처리용 등순도시험
(1) 염화물 : 이 품목 2g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.3mL에 대응하는 양 이하이어야 한다.
(2) 질산염 : 물 8mL에 이 품목 5g을 천천히 가하여 브루신황산용액(1→500) 1mL 및 황산을 가하여 25mL로 하여 잘 흔들어 섞고 약 80℃로 10분간 가온할 때, 그 액의 색은 질산염표준용액 0.5mL에 물 약 8mL를 가하여 황산 5mL를 천천히 가하고 브루신황산용액(1→500) 1mL 및 황산을 가하여 25mL로 하고 잘 흔들어 섞어 약 80℃로 10분간 가온한 액의 색보다 진하여서는 아니 된다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 이 품목 5.0g을 정밀히 달아 물을 가하여 25mL로 한 액을 시험용액으로 하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(5) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(6) 셀레늄 : 이 품목 0.3g을 정밀히 달아 4N 염산 25mL를 미리 가해준 150mL 비이커에 조심스럽게 넣고 섞은 다음 끓을 때 까지 가열하고 15분간 수욕에서 가열한 다음 물 25mL를 가해주고 냉각한 액을 시험용액으로 한다. 대조액은 표준용액 2mL를 비이커에 취하여 2N 염산 50mL로 희석한 액을 사용하고 공시험액은 2N 염산 50mL를 사용한다. 시험용액, 대조액 및 공시험액에 암모니아수 5mL를 각각 조심스럽게 가해주고 식힌 다음 각 용액을 암모니아수(1→2)를 사용하여 pH 1.8~2.2로 조정한다. 각 용액에 염산히드록실아민 0.2g을 가해주고 조심스럽게 흔들어 용해한 다음 바로 2,3-디아미노나프탈렌용액 5mL를 가하고 섞어준 다음 100분간 방치한다. 각 용액을 분액깔대기에 옮기고 물 10mL로 씻어 합한 다음 시클로헥산 5mL로 추출한다. 물층을 버리고 시클로헥산층을 원심분리하여 미량의 물을 제거한 다음 파장 380nm에서 흡광도를 측정할 때, 시험용액의 흡광도는 대조액의 흡광도보다 커서는 아니 된다(20ppm 이하).
표준용액 : 셀레늄표준용액을 물로 희석하여 3ppm으로 한다.
2,3-디아미노나프탈렌용액 : 2,3-디아미노나프탈렌 0.1g과 염산히드록실아민 0.5g을 0.1N 염산에 녹여 100mL로 한다.
(7) 철 : 이 품목 5.0g을 정밀히 달아 물을 가하여 25mL로 한 액을 시험용액으로 하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 20ppm 이하이어야 한다.
(8) 산화되기 쉬운 물질 : 물 10mL에 이 품목 8g을 가하여 식힌 다음 0.1N 과망간산칼륨용액 0.1mL를 가할 때, 이 액의 홍색은 5분 이내에 없어져서는 아니 된다.
(9) 환원성물질 : 이 품목 8g을 얼음물 50mL에 조심스럽게 희석하고 0.1N 과망간산칼륨용액 0.1mL를 가할 때, 나타내는 홍색은 5분 이내에 사라져서는 아니 된다(이산화황으로서 40ppm 이하).
확인시험
(1) 이 품목의 수용액(1→100)은 강산성이다.
(2) 이 품목의 수용액(1→100)은 확인시험법 중 황산염의 반응을 나타낸다.
정량법
이 품목 약 2g을 정밀히 달아 물 약 50mL 중에 가하고 식힌 다음 물을 가하여 100mL로 하고 그 중 25mL를 취하여 0.5N 수산화나트륨용액으로 적정한다(지시약 : 브로모티몰블루시액 1~2방울).
0.5N 수산화나트륨용액 1mL = 24.52mg H2SO4
강열잔류물
이 품목 5g을 백금접시 또는 석영접시에 취하여 수욕상에서 증발건고한 다음 항량이 될 때까지 450~550℃로 강열할 때, 그 잔류물은 1mg 이하이어야 한다.
개요
ReactivitySulfuric acid is very reactive and dissolves most metals, it is a concentrated acid that oxidizes, dehydrates, or sulfonates most organic compounds, often causes charring. Sulfuric acid reacts violently with alcohol and water to release heat. It reacts with most metals, particularly when diluted with water, to form flammable hydrogen gas, which may create an explosion hazard. Sulfuric acid is not combustible, but it is a strong oxidizer that enhances the combustion of other substances, does not burn itself. During fire, poisonous gases are emitted. Hazardous decomposition products are as follows: sulfur dioxide, sulfur trioxide, and sulfuric acid fumes. Note: Use great caution in mixing with water due to heat release that causes explosions. Always add the acid to water, never the reverse.
Where Found
l Car battery acid
l Certain detergents
l Chemical munitions
l Some fertilizers
l Some toilet bowl cleaners
Derivation
Sulfuric acid is made from sulfur, pyrite (FeS2), hydrogen sulfide, or sulfur-containing smelter gases by the contact process (vanadium pentoxide catalyst). The first step is combustion of elemental sulfur, or roasting of iron pyrites, to yield sulfur dioxide. Then follows the critical reaction, catalytic oxidation of sulfur dioxide to sulfur trioxide.
화학적 성질
Sulfuric acid is a colorless to dark brown, odorless, oily liquid which is commercially sold @ 93% to 98% H2SO4, the remainder being water.역사
Sulfuric acid is a colorless, oily, dense liquid that is one of the most important industrial chemicals. More than 40 million tons are produced in the United States annually and approximately 170 million tons are produced globally. Sulfuric acid has a long history and was first produced by ancient alchemists. Its discovery is credited to the Persian physician Mohammad Ibn Zakariya al-Razi (Rhazes, 854 925), who produced sulfuric acid from the dry distillation of minerals. Dry distillation typically involves heating a substance in a closed container to limit oxygen and combustion. As the substance is heated, it decomposes and the volatile components can be captured. Because sulfuric acid was obtained from distilling minerals, it is called a mineral acid. The ancient method of sulfuric acid production involved heating either iron (II) sulfate heptahydrate (FeSO4 7H2O), which was called green vitriol, or copper (II) sulfate pentahydrate (CuSO4 5H2O), called blue vitriol. When minerals containing these compounds were heated, the products included sulfur trioxide (SO3) and water. The combination of sulfur trioxide and water produced sulfuric acid: SO3(g) + H2O(l) H2SO4(aq). The production of sulfuric acid from natural minerals called vitriols and its oily appearance led to the common name oil of vitriol for sulfuric acid.용도
Sulfuric Acid is an acidulant that is a clear, colorless, odorless liquid with great affinity for water. it is prepared by reacting sulfur dioxide with oxygen and mixing the resulting sulfur trioxide with water, or by reacting nitric oxide with sulfur dioxide in water. it is very cor- rosive. it is used as a modifier of food starch and is used in caramel production and in alcoholic beverages.생산 방법
Sulfuric acid may be prepared industrially by either the contact process or the chamber process.
Contact Process
2SO2+O2→2SO3
SO3+H2O→H2SO4
Chamber Process
2NO+O2→2NO2
NO2+SO2+H2O→H2SO4+NO
일반 설명
Sulphuric acid may be prepared by catalytic oxidation of sulphur dioxide. It is a very strong electrolyte and has high affinity to water.공기와 물의 반응
Reaction with water is negligible unless acid strength is above 80-90% then heat from hydrolysis is extreme, may cause severe burns [Merck, 11th ed. 1989]. During sulfonation of mononitrobenzene by fuming Sulfuric acid , a leak from an internal cooling coil permitted water to enter the reaction tank. A violent eruption occurred due to the heat of solution [MCA Case History 944 1963].위험도
Strong irritant to tissue. Pulmonary function inhibitor. Confirmed carcinogen.건강위험
Concentrated sulfuric acid is a highly corrosive liquid that can cause severe, deep burns upon skin contact. The concentrated acid destroys tissue because of its dehydrating action, while dilute H 2SO4 acts as a skin irritant because of its acid character. Eye contact with concentrated H2SO4 causes severe burns, which can result in permanent loss of vision; contact with dilute H2SO4 results in more transient effects from which recovery may be complete. Sulfuric acid mist severely irritates the eyes, respiratory tract, and skin. Because of its low vapor pressure, the principal inhalation hazard from sulfuric acid involves breathing in acid mists, which may result in irritation of the upper respiratory passages and erosion of dental surfaces. Higher inhalation exposures may lead to temporary lung irritation with difficulty breathing. Ingestion of sulfuric acid may cause severe burns to the mucous membranes of the mouth and esophagus. Animal testing with sulfuric acid did not demonstrate carcinogenic, mutagenic, embryotoxic, or reproductive effects. Chronic exposure to sulfuric acid mist may lead to bronchitis, skin lesions, conjunctivitis, and erosion of teeth.화재위험
Sulfuric acid is highly reactive and capable of igniting finely-divided combustible materials on contact. When heated, Sulfuric acid emits highly toxic fumes. Avoid heat; water and organic materials. Sulfuric acid is explosive or incompatible with an enormous array of substances. Can undergo violent chemical change at elevated temperatures and pressure. May react violently with water. When heated, Sulfuric acid emits highly toxic fumes. Hazardous polymerization may not occur.인화성 및 폭발성
Sulfuric acid is noncombustible but can cause finely divided combustible substances to ignite. Sulfuric acid reacts with most metals, especially when dilute, to produce flammable and potentially explosive hydrogen gas.Pharmaceutical Applications
Sulfuric acid is used as an acidifying agent in a variety of pharmaceutical and food preparations. It may also be used to prepare dilute sulfuric acid, which, in addition to its use as an excipient, has some therapeutic use for the treatment of gastric hypoacidity, as an astringent in diarrhea, or to stimulate appetite. Sulfuric acid has been used in parenteral, oral, topical, and ophthalmic pharmaceutical formulations.공업 용도
Sulfuric acid (H2SO4) is the most widely used acid for pH control in mineral flotation. Sulfuric acid can be manufactured by several processes including the burning of pure sulfur, roasting of pyrite and from the recovery of SO2 stack gas from a smelter operation. Sulfuric acid is a colorless to amber, slightly cloudy and oily liquid with a specific gravity of 1.84 at 95% strength. In mineral flotation, sulfuric acid is used in almost all applications involving acid pH control. It is also used as a pulp pretreatment chemical during flotation of oxidic and industrial minerals. Pulp pretreatment with sulfuric acid improves flotation of ilmenite, perovskite, phenacite, beryl and other minerals.Safety
Sulfuric acid is widely used in a variety of pharmaceutical formulations. Although concentrated sulfuric acid is very corrosive, it is normally used well diluted in formulations. Concentrated sulfuric acid will react violently with water and much heat is generated. When diluting sulfuric acid, the acid should always be added to the other liquid with great caution.The concentrated solution is extremely corrosive and can cause severe damage or necrosis on contact with the eyes and skin. Ingestion may cause severe injury or death. Inhalation of concentrated vapors can cause serious lung damage.
LD50 (rat, oral): 2.14 g/kg
잠재적 노출
Used as a chemical feedstock in the manufacture of acetic acid, hydrochloric acid; citric acid; phosphoric acid; aluminum sulfate; ammonium sulfate;barium sulfate; copper sulfate; phenol, superphosphates, titanium dioxide; as well as synthetic fertilizers, nitrate explosives; artificial fibers; dyes, pharmaceuticals, detergents, glue, paint, and paper. It finds use as a dehydrating agent for esters and ethers due to its high affinity for water; as an electrolyte in storage batteries; for the hydrolysis of cellulose to obtain glucose; in the refining of mineral and vegetable oil; and in the leather industry. Other uses include fur and food processing; carbonization of wool fabrics; gas drying; uranium extraction from pitchblende; and laboratory analysis. Sulfuric acid is among the highestvolume produced chemical in the United States.Carcinogenicity
Strong inorganic acid mists containing sulfuric acid are known to be human carcinogens based on sufficient evidence of carcinogenicity from studies in humans.환경귀착
Although sulfuric acid can be extremely harmful, it is a naturally occurring compound. The release of sulfur into the biosphere is not from anthropogenic sources. It is also a major compound that is released in volcanic eruptions when oxides of sulfur are emitted:Sulfur trioxide will dissolve in rainwater to form sulfuric acid SO3 + H2O → H2SO4:
Sulfur dioxide will dissolve in rainwater to form sulfurous acid (H2SO3), and is then oxidized to form sulfuric acid, which leads to acid rains.
The presence of sulfuric acid is related with the natural ability of microorganisms that can be found in or isolated from acid mine water or from sulfur and iron sulfide mines as well as volcanoes.
The examples of such bacteria are:
Acidithiobacillus ferrooxidans (Thiobacillus ferrooxidans) that lives in pyrite deposits, metabolizing iron and sulfur and producing sulfuric acid.
Acidithiobacillus thiooxidans (Thiobacillus thiooxidans, Thiobacillus concretivorus) that utilizes sulfur and produces sulfuric acid.
저장
Splash goggles and rubber gloves should be worn when handling this acid, and containers of sulfuric acid should be stored in a wellventilated location, separated from organic substances and other combustible materials. Containers of sulfuric acid should be stored in secondary plastic trays to avoid corrosion of metal storage shelves due to drips or spills. Water should never be added to sulfuric acid because splattering may result; always add acid to water운송 방법
UN1830 Sulfuric acid with >51% acid or sulfuric acid with not >51% acid, Hazard class: 8; Labels: 8-Corrosive material. UN1831 Sulfuric acid, fuming with 30% or more free sulfur trioxide and Sulfuric acid, fuming, with <30% free sulfur trioxide, Hazard class: 8; Labels: 8-Corrosive material. UN1832 Sulfuric acid, spent, Hazard class: 8; Labels: 8-Corrosive material.Purification Methods
Sulfuric acid, and also 30% fuming H2SO4, can be distilled in an all-Pyrex system, optionally from potassium persulfate. It has been purified by fractional crystallisation of the monohydrate from the liquid. It has a very strong dehydrating action and attacks skin—wash immediately with cold H2O; otherwise the skin can be scarred for life. It is very hygroscopic and has been used as a desiccant in desiccators. Dilution with H2O is highly exothermic, and because the concentrated acid is much more dense than H2O it is diluted by running the concentrated acid down the side of the container of H2O with slowly stirring while cooling the outside of the container. If these precautions are not taken, the H2O is likely to boil vigorously.비 호환성
Avoid storage in close proximity to water, most common metals, organic materials, strong reducing agents, combustible materials, strong bases, carbonates, sulfides, cyanides, strong oxidizing agents, and carbides.Sulfuric acid is a powerful oxidizer and may ignite or explode on contact with many materials.
It can react violently with the evolution of a large amount of heat. Oxides of sulfur and hydrogen can be generated during reactions.
Great care must be exercised when mixing with other liquids. Always add sulfuric acid to the diluent with great caution.
폐기물 처리
Add slowly to solution of soda ash and slaked lime with stirring; flush to drain with large volumes of water. Recovery and reuse of spent sulfuric acid may be a viable alternative to disposal, and processes are available.Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM, IV, and IP injections, inhalation solutions, irrigation solutions, nasal, ophthalmic solutions and suspensions, oral solutions, and topical emulsions and creams). Included in nonparenteral and parenteral medicines licensed in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients.The United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances (1988) lists sulfuric acid as a chemical frequently used in the illicit manufacture of narcotic drugs or psychotropic substances. In the USA, sulfuric acid is included in the list of essential or precursor chemicals established pursuant to the Chemical Diversion and Trafficking Act. Accordingly, transactions of sulfuric acid such as imports, exports, sales, and transfers are subject to regulation and monitoring by the Drug Enforcement Administration.