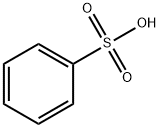
벤젠술폰 산
|
|
벤젠술폰 산 속성
- 녹는점
- 30-60 °C
- 끓는 점
- 137℃
- 밀도
- 1.32
- 증기압
- 69.8Pa at 20℃
- 굴절률
- 1.5151 (estimate)
- 인화점
- >230 °F
- 저장 조건
- Store below +30°C.
- 용해도
- H2O: 용해성0.1g/10mL, 투명, 무색
- 물리적 상태
- 축축한 결정질 고체 또는 융합된 덩어리
- 산도 계수 (pKa)
- 0.7(at 25℃)
- 색상
- 노란색에서 밝은 갈색까지
- 수소이온지수(pH)
- 2 (H2O, 20℃)(saturated solution)
- 수용성
- 녹는
- 감도
- Hygroscopic
- Merck
- 14,1070
- BRN
- 742513
- 안정성
- 안정적인. 강한 산화제, 염기, 많은 유기 화합물과 호환되지 않습니다.
- InChIKey
- SRSXLGNVWSONIS-UHFFFAOYSA-N
- LogP
- 0.41 at 25℃
- CAS 데이터베이스
- 98-11-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-34 | ||
| 안전지침서 | 26-36/37/39-45-36 | ||
| 유엔번호(UN No.) | UN 2583 8/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | DB4200000 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 29041000 | ||
| 유해 물질 데이터 | 98-11-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 1175 mg/kg | ||
| 기존화학 물질 | KE-02594 |
벤젠술폰 산 C화학적 특성, 용도, 생산
용도
1) 페 놀 및 resorcinol 제조에 사용. 2) 막힘의 형성을 리프트 수 유전 물 분사에 대 한 사용, 형성 침투성을 개선. 3) 파운드리 산업에 대 한 경화제와 에스테 르 화와 탈수 반응 위한 촉매로 사용.제품 소개
크리스탈 화이트. 물, 에탄올, 에테르와 벤젠에 약간 녹는 이황화 탄소에 불용 성에 쉽게 용 해.개요
Benzene sulfonic acid is an organo sulfur compound with the formula C6H5SO3H. It is the simplest aromatic sulfonic acid. It forms colorless deliquescent sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in benzene and insoluble in carbon disulfide and diethyl ether. It is often stored in the form of alkali metal salts. Its aqueous solution is strongly acidic.화학적 성질
green solid역사
Benzenesulfonic acid was first obtained, together with diphenyl sulfone, by E. M ITSCHERLICH in 1834 by heating benzene with fuming sulfuric acid. The industrially important reaction of benzenesulfonic acid with alkali hydroxide to form phenol (alkali fusion) was developed by A. WURTZ and A. KEKULé in 1867 and by P. O. D EGENER in 1878. Until the early 1960s benzenesulfonic acid was used chiefly in the manufacture of phenol. Other phenol syntheses are preferred now.용도
Benzenesulfonic acid is mainly consumed by conversion to other specialty chemicals. A variety of pharmaceutical drugs are prepared as salts of benzenesulfonic acid and are known as besylates or besilates. The alkali metal salt of benzenesulfonic acid was once widely used in the production of phenol. It acts as an acid catalyst for direct esterification of amino acids and peptides.주요 응용
The alkali metal salt of benzene sulfonic acid was once widely used in the production of phenol :C6H5SO3Na + 2 NaOH → C6H5ONa + Na2SO3
C6H5ONa + HCl → C6H5OH + NaCl
The process has been largely displaced by the Hock process, which generates less waste. Benzene sulfonic acid is mainly consumed by conversion to other specialty chemicals. A variety of pharmaceutical drugs are prepared as salts of benzene sulfonic acid and are known as besylates or besilates.
제조 방법
Benzene sulfonic acid is prepared from the sulfonation of benzene using concentrated sulfuric acid :This conversion illustrates aromatic sulfonation, which has been called "one of the most important reactions in industrial organic chemistry.".
정의
ChEBI: The simplest member of the class of a benzenesulfonic acids that consists of a benzene carrying a single sulfo group.주요 응용
Benzenesulfonic acids are used chiefly as intermediates. They are employed in the manufacture of sulfonic acid amides, hydrazides, and esters; of sulfinic acids, sulfones, phenols, and thiophenols; and of other compounds. Sulfonic acids that are substituted with OH and/or NH 2 groups serve as intermediates in the manufacture of finishing agents, optical brighteners, pickling agents, dyes, tanning agents, water-soluble resins, insecticides, ion-exchange resins, wetting agents, pharmaceuticals, polymeric thickeners, plasticizers, etc. Benzenesulfonic acids are also used as such as acidic catalysts and standardizing agents in dye manufacture.화학 반응
Benzene sulfonic acid exhibits the reactions typical of other aromatic sulfonic acids, forming sulfonamides , sulfonyl chloride, and esters. The sulfonation is reversed above 220 °C. Dehydration with phosphorus pentoxide gives benzene sulfonic acid anhydride ((C6H5SO2)2O). Conversion to the corresponding benzene sulfonyl chloride (C6H5SO2Cl) is effected with phosphorus penta chloride. It is a strong acid, being dissociated in water.일반 설명
Deliquescent needles or large plates.공기와 물의 반응
Slightly soluble in water.반응 프로필
Benzenesulfonic acid reacts with bases and many organic compounds. Benzenesulfonic acid has the characteristic reactions of a strong aromatic sulfonic acid. Acid hydrolysis at 175 ℃ splits it into benzene and sulfuricacid. Additional sulfonation with fuming sulfuric acid gives 1,3-benzenedisulfonic acid, which reacts further to 1,3,5-benzenetrisulfonic acid, and also diphenyl sulfone disulfonic acid.Benzenesulfonic acid reacts with benzene to form diphenyl sulfone according to a Friedel – Crafts-type reaction.
Benzenesulfonic acid reacts with alkali metal hydroxide at 320 – 350 ℃ to form sodium phe- nolate. This reaction was used in the first industrial synthesis of phenol.
화재위험
Flash point data for Benzenesulfonic acid are not available, however Benzenesulfonic acid is probably combustible.Safety Profile
Poison by ingestion, sbn contact, and probably inhalation. A severe skin and eye irritant. See also SULFATES and SULFONATES.Purification Methods
Purify benzenesulfonic acid by dissolving it in a small volume of distilled H2O and stirring with slightly less than the theoretical amount of BaCO3. When effervescence is complete and the solution is still acidic, filter off the insoluble barium benzenesulfonate. The salt is collected and dried to constant weight in vacuo, then suspended in H2O and stirred with a little less than the equivalent (half mol.) of sulfuric acid. The insoluble BaSO4 (containing a little barium benzenesulfonate) is filtered off and the filtrate containing the free acid is evaporated in a high vacuum. The oily residue will eventually crystallise when completely anhydrous. A 32% commercial acid is allowed to fractionally crystallise at room temperature over P2O5 in a vacuum desiccator giving finally colourless deliquescent plates m 52.5o. The anhydrous crystalline acid is deliquescent and should be stored over anhydrous Na2SO4 in the dark and should be used in subdued sunlight as it darkens under sunlight. The main impurity is Fe which readily separates as the Fe salt in the early fractions [Taylor & Vincent J Chem Soc 3218 1952]. The S-benzylisothiuronium salt has m 148o (from EtOH/H2O). It is an IRRITANT to the skin and eyes. [See Adams & Marvel Org Synth Coll Vol I 84 1941, Michael & Adair Chem Ber 10 585 1877, Beilstein 11 IV 27.]벤젠술폰 산 준비 용품 및 원자재
원자재
염화나트륨
아황산나트륨
Benzenesulfonic acid sodium salt
물
(2S)-1-(3-Acetylthio-2-methyl-1-oxopropyl)-L-proline
준비 용품
C.I 형광발색제 220
synthetic tanning agent No.29
광택이나는 BLUE R-250
9,9-dimethylcarbazine
디미듐 브로마이드
스테아르산
Acid Black 26
CIS-4-DECENAL
에톡시퀸
LANASOLBLUE3R
N-Phospho-L-arginine
4,4'-메틸렌디아닐린
바닐린
암모늄 비카보네이트
부틸벤젠술폰아미드
diethyl 2,6-bis((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
AtracuriuM IMpurity D2 (cis-Quaternary Alcohol)
디페닐술폰
TRIMETHYLSILYLBENZENESULFONATE
벤젠술폰 산 공급 업체
글로벌( 414)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 147 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |