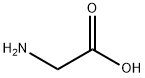
글리신
|
|
글리신 속성
- 녹는점
- 240 °C (dec.) (lit.)
- 끓는 점
- 233°C
- 밀도
- 1.595
- 증기압
- 0.0000171 Pa (25 °C)
- 굴절률
- 1.4264 (estimate)
- FEMA
- 3287 | GLYCINE
- 인화점
- 176.67°C
- 저장 조건
- 2-8°C
- 용해도
- H2O: 100 mg/mL
- 물리적 상태
- 가루
- 산도 계수 (pKa)
- 2.35(at 25℃)
- 색상
- <5(200mg/mL)(APHA)
- 수소이온지수(pH)
- 4(0.2 molar aqueous solution)
- 냄새
- 냄새 없는
- pH 범위
- 4
- ?? ??
- 냄새 없는
- 수용성
- 25g/100mL(25℃)
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.05
λ: 280 nm Amax: 0.05
- JECFA Number
- 1421
- Merck
- 14,4491
- BRN
- 635782
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제와 호환되지 않습니다.
- InChIKey
- DHMQDGOQFOQNFH-UHFFFAOYSA-N
- LogP
- -3.21
- CAS 데이터베이스
- 56-40-6(CAS DataBase Reference)
- NIST
- Glycine(56-40-6)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험 카페고리 넘버 | 33 | ||
|---|---|---|---|
| 안전지침서 | 22-24/25 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | MB7600000 | ||
| TSCA | Yes | ||
| HS 번호 | 29224910 | ||
| 유해 물질 데이터 | 56-40-6(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 7930 mg/kg | ||
| 기존화학 물질 | KE-01153 |
글리신 C화학적 특성, 용도, 생산
개요
글라이신(Glycine, 글리신)은 HO2CCH2NH2의 화학식을 갖는 유기물이다. 글라이신은 20개의 기본 아미노산 중의 하나로 동물 단백질에서 흔히 발견된다.용도
생체합성 중간물질로서의 역할.
글라이신은 수많은 종에서 구성물질로 쓰인다.
글라이신은 중추 신경계, 특히 척수, 뇌간, 시신경의 중추신경계에서 신경전달억제물질이다.
합성
글라이신은 인체에서 합성될 수 있기 때문에 비필수아미노산에 속한다. 세린과 엽산이 반응해서 CH2-엽산과 물을 만들고 수소만 남기는 반응인데, 이 반응은 세린 하이드록시메틸 전이효소에 의해서 촉매된다.순도시험
(1) 용상 : 이 품목 1g을 물 10mL에 녹일 때, 그 액은 무색 징명하여야 한다.
(2) 액성 : 이 품목의 수용액(1→10)의 pH는 5.5~7.0이어야 한다.
(3) 염화물 : 이 품목 0.5g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.3mL에 대응하는 양 이하이어야 한다.
(4) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(5) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
(6) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
확인시험
(1) 이 품목의 수용액(1→10) 5mL에 묽은염산 5방울 및 아질산나트륨시액 1mL를 가하면 무색의 가스를 발생한다.
(2) 위 (1)의 반응을 끝낸 액 5방울을 작은 시험관에 취하고 잠시 동안 끓이고 계속하여 120℃의 건조기 중에서 증발건고하여 식힌 다음 잔류물에 크로모트로프산시액 5~6방울을 가하고 수욕 중에서 10분간 가열하면 액은 진한 자색을 나타낸다.
(3) 이 품목의 수용액(1→1,000) 5mL에 닌히드린시액 1mL를 가하여 3분간 가열하면 자색을 나타낸다.
정량법
이 품목 약 0.15g을 정밀히 달아 개미산 3mL에 녹이고 빙초산 50mL를 가하여 0.1N 과염소산용액으로 적정한다(지시약 : α-나프톨벤제인시액 0.5mL). 종말점은 액의 갈색이 녹색으로 변하는 점이다.
따로 같은 방법으로 공시험을 한다.
0.1N 과염소산용액 1mL = 7.507mg C2H5O2N
강열잔류물
이 품목 1g을 취하여 강열잔류물시험법에 따라 시험할 때, 그 양은 0.1% 이하이어야 한다.
개요
Glycine (abbreviated as Gly or G) is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its side-chain, glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG of the genetic code.Glycine is a colourless, sweet-tasting crystalline solid. It is unique among the proteinogenic amino acids in that it is not chiral. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. Glycine is also the genus name of the Soybean plant (species name = Glycine max).
화학적 성질
Glycine occurs as a white, odorless, crystalline powder, and has a sweet taste.출처
Gelatin and silk fbroin are reportedly the best natural sources of this amino acid용도
Glycine is a non-essential amino acid for human development. Glycine is an inhibitory neurotransmitter in spinal cord, allosteric regulator of NMDA receptors.정의
ChEBI: The simplest (and the only achiral) proteinogenic amino acid, with a hydrogen atom as its side chain.제조 방법
From chloroacetic acid and ammonia; from protein sources, such as gelatin and silk fbroin; from ammonium bicarbonate and sodium cyanide; by catalytic cleavage of serine; from hydrobromic acid and methyleneaminoacetonitrile.생산 방법
Chemical synthesis is the most suitable method of preparation of glycine. Amination of chloroacetic acid and the hydrolysis of aminoacetonitrile are the favored methods of production.Biosynthesis
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phospho glycerate. In most organisms, the enzyme Serine hydroxy methyl transferase catalyses this transformation via the cofactor pyridoxal phosphate :serine + tetra hydro folate → glycine +N5,N10-Methylene tetrahydrofolate + H2O
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible : CO2 + NH4+ + N5,N10-Methylene tetra hydro folate + NADH + H+→ Glycine + tetrahydrofolate +NAD+
Glycine is coded by codons GGU, GGC, GGA and GGG. Most proteins incorporate only small quantities of glycine. A notable exception is collagen, which contains about 35 % glycine.
Biological Functions
The principal function of glycine is as a precursor to proteins. It is also a building block to numerous natural products.As a biosynthetic intermediate
In higher eukaryotes, D-Aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA. Glycine provides the central C2N subunit of all purines.
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potentia (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required coagonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutaminergic receptors which are excitatory. The LD50 of glycine is 7930 mg / kg in rats (oral), and it usually causes death by hyperexcitability. .
일반 설명
White crystals.공기와 물의 반응
Water soluble.반응 프로필
An amino acid. A 0.2M aqueous solution has a pH of 4.0., so acts as a weak acid. Has characteristics of both acid and base.위험도
Use in fats restricted to 0.01%.화재위험
LOW. Ignites at very high temperatures.농업용
Glycine is the simplest naturally occurring amino acid and is a constituent of most proteins. Its formula is H2N·CH2·COOH.Pharmaceutical Applications
Glycine is routinely used as a cofreeze-dried excipient in protein formulations owing to its ability to form a strong, porous, and elegant cake structure in the final lyophilized product. It is one of the most frequently utilized excipients in freeze-dried injectable formulations owing to its advantageous freeze-drying properties.Glycine has been investigated as a disintegration accelerant in fast-disintegrating formulations owing to its excellent wetting nature.It is also used as a buffering agent and conditioner in cosmetics.
Glycine may be used along with antacids in the treatment of gastric hyperacidity, and it may also be included in aspirin preparations to aid the reduction of gastric irritation.
생물학적 활성
One of the major inhibitory neurotransmitters in the mammalian CNS, predominantly active in the spinal cord and brain stem. Also acts as a modulator of excitatory amino acid transmission mediated by NMDA receptors. Also available as part of the NMDA Receptor - Glycine Site Tocriset™ .Safety Profile
Moderately toxic by intravenous route. Mildly toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.Safety
Glycine is used as a sweetener, buffering agent, and dietary supplement. The pure form of glycine is moderately toxic by the IV route and mildly toxic by ingestion.Systemic absorption of glycine irrigation solutions can lead to disturbances of fluid and electrolyte balance and cardiovascular and pulmonary disorders.
LD50 (mouse, IP): 4.45 g/kg
LD50 (mouse, IV): 2.37 g/kg
LD50 (mouse, oral): 4.92 g/kg
LD50 (mouse, SC): 5.06 g/kg
LD50 (rat, IV): 2.6 g/kg
LD50 (rat, oral): 7.93 g/kg
LD50 (rat, SC): 5.2 g/kg
저장
Glycine starts to decompose at 233°C. Store in well-closed containers. Glycine irrigation solutions (95–105% glycine) should be stored in single dose containers, preferably type I or type II glass.Purification Methods
Crystallise glycine from distilled water by dissolving at 90-95o, filtering, cooling to about -5o, and draining the crystals centrifugally. Alternatively, crystallise it from distilled water by addition of MeOH or EtOH (e.g. 50g dissolved in 100mL of warm water, and 400mL of MeOH is added). The crystals are washed with MeOH or EtOH, then with diethyl ether. Likely impurities are ammonium glycinate, iminodiacetic acid, nitrilotriacetic acid or/and ammonium chloride. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 1955 1961, Beilstein 4 IV 2349.]비 호환성
Glycine may undergo Maillard reactions with amino acids to produce yellowing or browning. Reducing sugars will also interact with secondary amines to form an imine, but without any accompanying yellow-brown discoloration.Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM, IV, SC injections; oral; rectal) and approved for irrigant solutions. Included in parenteral (powders for injection; solutions for injection; vaccines; kits for implant) and nonparenteral (orodispersible tablets/oral lyophilizate; powders for inhalation; powders for oral solution; tablets) formulations licensed in the UK.글리신 준비 용품 및 원자재
원자재
준비 용품
글리신 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shijiazhuang Donghua Jinlong Chemical Co., LTD | +86-031166619888 +86-19333738986 |
donghuajinlong@glycine.com.cn | China | 25 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 7193 | 58 |
| Hebei ZB Gamay Biological Technology Co.,Ltd | +8617330018573 |
info@zbvet.net | China | 245 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 |
kyra@quanjinci.com | China | 1534 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 |
info@deshangchem.com | China | 656 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 |
admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 |
admin@86-ss.com | China | 1000 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5895 | 58 |
| Suzhou Sanyi Polymer Chemical Technology Co., Ltd. | +8615571922873 |
15571922873@163.com | China | 106 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |