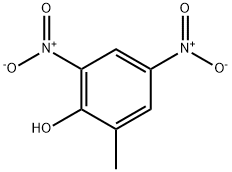
2-메틸-4,6-디니트로페놀
|
|
2-메틸-4,6-디니트로페놀 속성
- 녹는점
- 83-85 °C(lit.)
- 끓는 점
- 196°C 1012mm
- 밀도
- 1.5928 (rough estimate)
- 증기압
- 5.2(x 10-5 mmHg) at 25 °C (Melnikov, 1971)5(x 10-5 mmHg) at 20 °C (ACGIH, 1986)
- 굴절률
- 1.5460 (estimate)
- 인화점
- 11 °C
- 저장 조건
- 0-6°C
- 용해도
- 용해도 물에 거의 용해되지 않음; 에탄올, 아세톤, 에테르에 쉽게 용해됨
- 산도 계수 (pKa)
- 4.42(at 25℃)
- 색상
- 노란색
- pH 범위
- Colorless (2.4) to yellow (3.8)
- 수용성
- 약간 용해됨
- Merck
- 3279
- BRN
- 2054389
- Henry's Law Constant
- 1.4(x 10-6 atm?m3/mol) at 25 °C (gas stripping-UV spectrophotometry, Warner et al., 1987)
- 노출 한도
- NIOSH REL: TWA 0.2 mg/m3, IDLH 5 mg/m3; OSHA PEL: TWA 0.2 mg/m3.
- 주요 응용
- 폭발물, 살균제, 제초제, 살충제, 항종양제
- CAS 데이터베이스
- 534-52-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T+,N,T,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 26/27/28-38-41-43-44-50/53-68-40-39/23/24/25-23/24/25-11-52/53-51/53 | ||
| 안전지침서 | 36/37-45-60-61-16-7 | ||
| OEB | C | ||
| OEL | TWA: 0.2 mg/m3 [skin] | ||
| 유엔번호(UN No.) | UN 1598 6.1/PG 2 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | GO9625000 | ||
| 위험 등급 | 6.1(a) | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 534-52-1(Hazardous Substances Data) | ||
| 독성 | LD50 in rats (mg/kg): 25-40 orally, 200-600 dermally (Ben-Dyke) | ||
| IDLA | 5 mg/m3 | ||
| 기존화학 물질 | KE-23779 | ||
| 유해화학물질 필터링 | 97-1-39 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 디엔오시과 그 염류 및 그 중 하나를 1% 이상 함유한 혼합물 |
2-메틸-4,6-디니트로페놀 C화학적 특성, 용도, 생산
화학적 성질
yellow to yellow-green crystals or cryst. powder용도
2-Methyl-4,6-dinitrophenol can be used as dormant ovicidal spray for fruit trees (highly phytotoxic and cannot be used successfully on actively growing plants); herbicide; insecticide.생산 방법
o-Cresol is sulfonated in excess 75 % sulfuric acid to give the disulfonic acid. The sulfonation mass is diluted with water, and 2 equivalents of nitric acid are added at 70 ℃ to form the dinitro derivative. The product is separated while molten and washed with hot water.정의
ChEBI: A hydroxytoluene that is o-cresol carrying nitro substituents at positions 4 and 6.일반 설명
A yellow solid. Emits toxic oxides of nitrogen fumes when heated to decomposition. Toxic by skin absorption, inhalation or ingestion. Soluble in alcohol, acetone, ether and solutions of sodium or potassium hydroxides.공기와 물의 반응
Slightly soluble in water.건강위험
Extremely toxic material; probable oral lethal dose is 5-50 mg/kg in humans or between 7 drops and 1 teaspoonful for a 70 kg (150 lb.) person.화재위험
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.농업용
Herbicide, Fungicide, Pesticide: DNOC is widely used in agriculture as a herbicide and pesticide; it is also used in the dyestuff industry. Although 4,6-dinitro-o-cresol (DNOC) is no longer registered for use in the United States, it was used as a blossom-thinning agent on fruit trees and as a fungicide, insecticide, and miticides on fruit trees during the dormant season. It is used in mushroom houses to control foreign fungi; to kill locusts and other insects; and as a pre-harvest desiccant of potatoes and leguminous seed crops. DNOC is used as free radical polymerization inhibitor and agricultural chemical intermediate; widely used in agriculture as a herbicide and pesticide; Hence, individuals formulating or spraying the compound incur the highest risk of exposure to the compound. Not approved for use in EU countries. DNOC’s registration in the U.S. as a pesticide was canceled in 1991. Currently, there are 39 global suppliers.상품명
ANTINONIN®; ANTINONNIN®; ARBOROL®; DEGRASSAN®; DEKRYSIL®; DETAL®; DILLEX®; DINOC®; DINURANIA®; DITROSOL®; DNOC®[C]; EFFUSAN®; EFFUSAN 3436®; ELGETOL®; ELGETOL 30®; ELIPOL®; EXTRAR®; FLAVIN-SANDOZ®; HEDOLIT®; HEDOLITE®; K III®; K IV®; KREOZAN®; KREZOTOL 50®; LIPAN®; NEUDORFF DN 50®; NITROFAN®; PROKARBOL®; RAFEX®; RAFEX 35®; RAPHATOX®; SANDOLIN®; SANDOLIN A®; SELINON®; SINOX®; WINTERWASH®Safety Profile
Human poison by unspecified route. Experimental poison by ingestion, inhalation, skin contact, intraperitoneal, and intravenous routes. Human systemic effects by ingestion and inhalation: somnolence, headache, abnormal brain recordings from specific areas of the central nervous system, cardlac and gastrointestinal changes. Mutation data reported. An e~7e and skin irritant. Less toxic than the para form, but is still highly toxic. A pesticide. See also NITRO COMPOUNDS of AROMATIC HYDROCARBONS and other dinitrocresol entries.Carcinogenicity
In one chronic feeding study in rats DNOC did not cause an increased incidence of any type of tumor. DNOC was clastogenic, increasing the frequency of chromosomal aberrations both in vivo and in vitro. Conflicting results for mutagenicity have been obtained in bacterial assays.The 2003 ACGIH threshold limit valuetime- weighted average (TLV-TWA) for dinitro-o-cresol is 0.2mg/m3 with a notation for skin absorption.
환경귀착
Soil/Plant. In plants and soils, the nitro groups reduced to amino groups (Hartley and Kidd, 1987). When 4,6-dinitro-o-cresol was statically incubated in the dark at 25°C with yeast extract and settled domestic wastewater inoculum, no signi?cant biodegradation and necessary acclimation for optimum biooxidation within the 4-week incubation period was observed (Tabak et al., 1981).Chemical/Physical. 4,6-Dichloro-o-cresol will react with amines and alkali metals forming water-soluble salts which are indicative of phenols (Morrison and Boyd, 1971).