아세트산부틸
|
|
아세트산부틸 속성
- 녹는점
- -78 °C (lit.)
- 끓는 점
- 124-126 °C (lit.)
- 밀도
- 0.88 g/mL at 25 °C (lit.)
- 증기 밀도
- 4 (vs air)
- 증기압
- 15 mm Hg ( 25 °C)
- 굴절률
- n
20/D 1.394(lit.)
- FEMA
- 2174 | BUTYL ACETATE
- 인화점
- 74 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 5.3g/L
- 물리적 상태
- 액체
- Specific Gravity
- 0.883 (20/20℃)
- 색상
- ≤10(APHA)
- 냄새
- 특성; 기분 좋은 과일향(낮은 농도); 비잔류.
- 수소이온지수(pH)
- 6.2 (5.3g/l, H2O, 20℃)(External MSDS)
- 폭발한계
- 1.4-7.5%(V)
- Odor Threshold
- 0.016ppm
- ?? ??
- 미묘한
- 수용성
- 0.7g/100mL(20℃)
- 어는점
- -77.9℃
- 최대 파장(λmax)
- λ: 254 nm Amax: 1.0
λ: 260 nm Amax: 0.20
λ: 275 nm Amax: 0.04
λ: 300 nm Amax: 0.02
λ: 320-400 nm Amax: 0.01
- Merck
- 14,1535
- JECFA Number
- 127
- BRN
- 1741921
- Henry's Law Constant
- 5.79 at 37 °C (static headspace-GC, van Ruth et al., 2001)
- Dielectric constant
- 5.0(20℃)
- 노출 한도
- TLV-TWA 150 ppm (~710 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 200 ppm (~950 mg/m3); IDLH 10,000 ppm (NIOSH).
- 안정성
- 안정적인. 가연성. 강한 산화제, 강산, 강염기와 호환되지 않습니다.
- InChIKey
- DKPFZGUDAPQIHT-UHFFFAOYSA-N
- LogP
- 1.82-2.3 at 25℃
- CAS 데이터베이스
- 123-86-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험 카페고리 넘버 | 10-66-67-R67-R66-R10 | ||
|---|---|---|---|
| 안전지침서 | 25-S25 | ||
| 유엔번호(UN No.) | UN 1123 3/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | AF7350000 | ||
| 자연 발화 온도 | 790 °F | ||
| TSCA | Yes | ||
| HS 번호 | 2915 33 00 | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| 유해 물질 데이터 | 123-86-4(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 14.13 g/kg (Smyth) | ||
| IDLA | 1,700 ppm | ||
| 기존화학 물질 | KE-04179 |
아세트산부틸 C화학적 특성, 용도, 생산
용도
1. 액체 부틸 아세테이트 (Liquid Butyl Acetate) 는 니트로 셀룰로오스 니스에 널리 사용되는 우수한 유기 용제이며 인조 가죽, 직물 및 플라스틱 가공, 향수 업계에도 사용됨. 2. GB 2760 96 규정에서 액체 부틸 아세테이트 는 향료를 사용하기위한 것이다. 3. 액체 부틸 아세테이트 가 과일 풍미에서 사용 되더라도, 주요로는 확산의 좋은 성능을 취하고, 사용하기에 더 적합합니다. 4. 부틸 아세테이트 는 우수한 유기 용제, 셀룰로스 아세테이트 부티레이트, 에틸 셀룰로오스, 염화 고무, 폴리스티렌, 메타 크릴 수지 및 껌, 마닐라 고무, 댐코 (Damco) 수지 등과 같은 많은 천연 수지가 양호한 용해도를 나타냅니다. 인조 가죽, 직물 및 플라스틱의 가공에서 용제로서 니트로 셀룰로오스 바니쉬에 널리 사용되며, 다양한 석유 가공 및 제약 공정 에서뿐만 아니라 향신료와 살구, 바나나, 배, 파인애플 등의 합성. 다양한 방향 성분. 5. 부틸 아세테이트 는 껌 로진, 폴리 비닐 아세테이트, 폴리 아크릴 레이트, 폴리 비닐 클로라이드, 염화 고무, 폴리 염화 비닐, 바니시, 인조 가죽, 의약, 플라스틱 및 향수 산업, (Eucommia gum), 폴리 메틸 메타 크릴 레이트 (polymethyl methacrylate) 등이있다. 6. 부틸 아세테이트 는 분석 시약, 크로마토 그래피 표준 물질 및 용매로 사용됩니다.용도
아세트산 n- 부틸은 쾌적한 과일 냄새가 나는 무색의 인화성 액체입니다. n- 부틸 아세테이트의 가장 일반적인 용도는 래커와 도료의 제조시 용매로 사용됩니다. 그것의 다른 주요 용도는 접착제 및 경화 된 코팅의 생성에 있습니다. 일반 부틸 아세테이트는 또한 제약 산업에서 용매 또는 추출 제로서 사용된다.용도
n- 부틸 아세테이트의 가장 보편적 인 사용은 래커와 도료의 생산에서 용매로서 사용됩니다. 그것의 다른 주요 용도는 접착제 및 경화 된 코팅의 생성에 있습니다. 일반 부틸 아세테이트는 또한 제약 산업에서 용매 또는 추출 제로서 사용된다.순도시험
(1) 비중 : 이 품목의 비중은 0.876~0.880이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 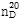 은 1.393~1.395이어야 한다.
은 1.393~1.395이어야 한다.
(3) 용상 : 이 품목 2mL를 70% 에탄올 4mL에 녹일 때, 그 액은 징명하여야 한다.
(4) 산가 : 이 품목의 산가는 향료시험법 중 산가측정법에 따라 시험할 때, 1.0 이하이어야 한다.
확인시험
이 품목 1mL에 10% 알콜성수산화칼륨용액 5mL를 넣어 수욕중에서 가열하면 특이한 향기는 없어지고 부탄올 냄새가 남는다. 식힌 다음 이에 물 10mL 및 묽은초산 0.5mL를 넣은 액은 확인시험법 중 초산염 (다)의 반응을 나타낸다.
정량법
이 품목 약 0.5g을 정밀히 달아 향료시험법 중 에스테르가 및 에스테르함량측정법에 따라 시험한다.
0.5N 알콜성수산화칼륨용액 1mL = 58.08mg C6H12O2
포장, 보관 및 운송
저장 및 운송 : 시원하고 건조하고 통풍이 잘되는 곳에 보관하십시오. 열에 노출시키지 마십시오.개요
Butyl acetate is a clear, flammable ester of acetic acid that occurs in n-, sec-, and tert- forms (INCHEM, 2005). Butyl acetate isomers have a fruity, banana-like odor (Furia, 1980). Isomers of butyl acetate are found in apples (Nicholas, 1973) and other fruits (Bisesi, 1994), as a well as in a number of food products, such as cheese, coffee, beer, roasted nuts, vinegar (Maarse and Visscher, 1989). Butyl acetate is manufactured via esterification of the respective alcohol with acetic acid or acetic anhydride (Bisesi, 1994). N-butyl acetate is used as a solvent for nitrocellulose-based lacquers, inks, and adhesives. Other uses include manufacture of artificial leathers, photographic film, safety glass, and plastics (Budavari, 1996). Isomers of butyl acetate are also used as flavoring agents, in manicure products, and as larvicides (Bisesi, 1994). The tert-isomer has been used as a gasoline additive (Budavari, 1996). It may be used as a synthetic fruit flavoring in candy, ice cream, cheeses, and baked goods (Dikshith, 2013).
화학적 성질
Butyl acetate is a colorless or yellowish liquid with a strong fruity odor. burning and then sweet taste reminiscent of pineapple. It occurs in many fruits and is a constituent of apple aromas. Butyl acetate is incompatible with strong oxidizing agents, strong acids, and strong bases.There are 4 isomers. At 20 °C, the density of the n-butyl isomer is 0.8825 g/ cm3, and the density of the sec-isomer is 0.8758 g/cm3 (Bisesi, 1994). The n-butyl isomer is soluble in most hydrocarbons and acetone, and it is miscible with ethanol, ethyl ether, and chloroform (Haynes, 2010). It dissolves many plastics and resins (NIOSH, 1981).
물리적 성질
Clear, colorless liquid with a strong fruity odor resembling bananas. Sweetish taste as low concentrations (<30 μg/L). Experimentally determined detection and recognition odor threshold concentrations were 30 μg/m3 (6.3 ppbv) and 18 μg/m3 (38 ppbv), respectively (Hellman and Small, 1974). Cometto-Mu?iz et al. (2000) reported nasal pungency threshold concentrations ranged from approximately 550 to 3,500 ppm.출처
Reported present in rum ether, pears, pear brandy, cider, mango, mountain papaya (C. pubescens), soybean, roasted peanuts and honey and other natural products.용도
Butyl Acetate is a flavoring agent which is a clear, colorless liquid possessing a fruity and strong odor. it is sparingly soluble in water and miscible in alcohol, ether, and propylene glycol. it is also termed n-butyl acetate.정의
ChEBI: Butyl acetate is the acetate ester of butanol. It has a role as a metabolite. It is functionally related to a butan-1-ol.제조 방법
By esterification of n-butyl alcohol with acetic acid.생산 방법
Butyl alcohol is combined with acetic acid in the presence of a catalyst such as sulfuric acid. After esterification is complete, the solution is distilled to yield butyl acetate .일반 설명
A clear colorless liquid with a fruity odor. Flash point 72 - 88°F. Density 7.4 lb / gal (less than water). Hence floats on water. Vapors heavier than air.공기와 물의 반응
Highly flammable. Very slightly soluble in water.반응 프로필
Butyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Attacks many plastics. [Handling Chemicals Safely 1980. p. 233].위험도
Skin irritant, toxic. Flammable, moderate fire risk. Eye and upper respiratory tract irritant.건강위험
Exposures to n-butyl acetate cause harmful effects that include, but are not limited to, coughing and shortness of breath. High concentrations have a narcotic effect, with symp toms such as sore throat, abdominal pain, nausea, vomiting, and diarrhea. High concen trations of n-butyl acetate cause severe poisoning. Prolonged periods of exposure cause adverse effects to the lungs, the nervous system, and the mucous membranes. Repeated skin contact causes skin dryness or cracking, and dermatitis.화재위험
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.Safety Profile
Moderately toxic by intraperitoneal route. Mdly toxic by inhalation and ingestion. An experimental teratogen. A skin and severe eye irritant. Human systemic effects by inhalation: conjunctiva irritation, unspecified nasal and respiratory system effects. A mild allergen. High concentrations are irritating to eyes and respiratory tract and cause narcosis. Evidence of chronic systemic toxicity is inconclusive. Flammable liquid. Moderately explosive when exposed to flame. Ignites on contact with potassium tert-butoxide. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid and irritating fumes. See also ESTERS.잠재적 노출
n-Butyl acetate is an important solvent in the production of lacquers, leather and airplane dopes, and perfumes. It is used as a solvent and gasoline additive. sec-Butyl acetate is used as a widely used solvent for nitrocellulose, nail enamels and many different purposes. tert-Butyl acetate is common industrial solvent used in the making of lacquers, artificial leather, airplane dope, perfume; and as a food additive. Isobutyl acetate is used as a solvent and in perfumes and artificial flavoring materialsCarcinogenicity
There are no indications of mutagenic or cytogenic effects for n-butyl acetate.저장
n-Butyl acetate should be kept stored in a segregated and approved area. Workers should keep the container in a cool, well-ventilated area, closed tightly, and sealed until ready for use. Workers should avoid all possible sources of ignition/spark at the workplace운송 방법
UN1123 Butyl acetates, Hazard Class: 3; Labels: 3—Flammable liquid.Purification Methods
Distil, reflux with successive small portions of KMnO4 until the colour persists, dry with anhydrous CaSO4, filter and redistil. [Beilstein 2 IV 143.]비 호환성
All butyl acetates are incompatible with nitrates, strong oxidizers; strong alkalies; strong acids. Butyl acetates may form explosive mixture with air; reacts with water, on standing, to form acetic acid and n-butyl alcohol. Violent reaction with strong oxidizers and potassium-tert-butoxide. Dissolves rubber, many plastics, resins and some coatings. May accumulate static electrical charges, and may cause ignition of its vapors폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.주의 사항
On exposure to Butyl acetate, immediately wash with plenty of water, also under the eyelids, for at least 15 min. Remove contact lenses. Butyl acetate is flammable in the pres ence of open flames, sparks, oxidizing materials, acids, and alkalis. It poses explosion risk in the presence of mechanical impact. For health safety, management authorities should provide exhaust ventilation facilities at the workplace to keep the airborne concentrations of vapors of Butyl acetate below TLV.아세트산부틸 준비 용품 및 원자재
원자재
준비 용품
Anethole trithione
아세틸 케텐
modified polyurethane seasoning agent
Acesulfame
Sineptina
폴리우레탄 중합체
2-METHOXY-6-METHYL-4(1H)-PYRIMIDINONE
seasoning agent GS-1
complex polystyrene high efficiency anticorrosion paint
4-Nitrophenyl-beta-D-galactopyranoside
페니실린 G 칼륨
PAINT
leather seasoning agent DLC-1
OXACILLIN SODIUM
4-NITROPHENYL-ALPHA-D-GLUCOPYRANOSIDE
4-NITROPHENYL-ALPHA-D-GALACTOPYRANOSIDE
scavenger of fabric maculae
에리트로마이신
아세토아세트산노르말부틸에스테르
티민
페니실린 감마-일라디산염 세포
톨루엔 디이소시안산 (혼합 이성질체)
메탄
Cephalothin sodium
4-NITROPHENYL-BETA-D-GLUCOPYRANOSIDE
아세트산부틸 공급 업체
글로벌( 1019)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 |
sales@yanshuochem.com | China | 101 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 |
sales01@sdlookchemical.com | China | 2739 | 58 |
| Hebei Duling International Trade Co. LTD | +8618032673083 |
sales05@hbduling.cn | China | 15745 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 |
peter68@ptchemgroup.com | China | 35453 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 991 | 58 |
| AS WATER CO., LTD. | +86-18994963526 +8618994963526 |
1346073549@qq.com | United Kingdom | 176 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |